16 July 2020: Animal Study
Calcitonin Gene-Related Peptide Attenuates LPS-Induced Acute Kidney Injury by Regulating Sirt1
Pingli Liu1ACDF, Dongmei Shi1ABEG*DOI: 10.12659/MSM.923900
Med Sci Monit 2020; 26:e923900
Abstract
BACKGROUND: Acute kidney injury (AKI) caused by sepsis is a very dangerous clinical complication. This study explored the effects of calcitonin gene-related peptides (CGRP) on AKI and its mechanisms.
MATERIAL AND METHODS: We cultured renal proximal tubular epithelial cells (HK-2 cells) and induced AKI models using LPS. Recombinant human CGRP was used to stimulate HK-2 cells and we detected markers of kidney injury (KIM-1 and NGAL) to determine the protective effect of CGRP on HK-2 cells. In addition, we constructed Sirt1-overexpressing lentivirus and small interfering RNA to increase or decrease Sirt1 expression in HK-2 cells to verify that CGRP protects HK-2 cells by regulating Sirt1.
RESULTS: After CGRP stimulation of HK-2 cells, LPS-induced HK-2 cell damage was significantly ameliorated, showing a decrease in the expression of KIM-1, NGAL, and inflammatory factors. In addition, Sirt1 was significantly increased in CGRP-stimulated HK-2 cells. After transfection of HK-2 cells with Lenti-Sirt1, inflammation and damage of HK-2 cells were both reduced, indicating that Sirt1 has a protective effect on HK-2 cells and can mediate the protective effect of CGRP on HK-2 cells. Therefore, the protective effect of CGRP on HK-2 cells was also attenuated after reducing Sirt1 in HK-2 cells. Finally, we used CGRP to treat LPS-induced mice and verified the protective effect of CGRP on mouse AKI.
CONCLUSIONS: CGRP has a significant anti-inflammatory effect. In the treatment of AKI, CGRP can increase the expression of Sirt1 to exert an anti-inflammatory effect and has a good protective effect on LPS-induced HK-2 cells.
Keywords: Acute Kidney Injury, Receptors, Calcitonin Gene-Related Peptide, Calcitonin, Calcitonin Gene-Related Peptide, Cell Line, Cytokines, Kidney, Kidney Tubules, Proximal, Lipopolysaccharides, Recombinant Proteins, Sepsis, Sirtuin 1
Background
Acute kidney injury (AKI) is a clinical syndrome with a sharp decline in kidney function that can be caused by a variety of factors. The disease is often accompanied by oliguria and symptoms such as azotemia, hyperkalemia, and metabolic acidosis [1]. AKI can be caused by a large number of causes, such as nephrotoxic drugs and renal ischemia reperfusion. These factors will cause renal cell necrosis and acute renal pathological changes, resulting in renal dysfunction. In particular, due to the increasing number of cancer cases in recent years, nephrotoxic anti-tumor drugs (such as cisplatin) have become important in increasing the incidence of AKI [2]. At present, the incidence of the disease is about 0.5% to 1% in the general population, and is 2% to 7% in hospitalized patients, and 4% to 25% in ICU and postoperative patients. In these groups, the mortality rate ranges from 28% to 90% [3]. In recent years, with the development of various medical technologies, many diseases have been effectively controlled. However, AKI has not been effectively treated, and the related mortality has not been significantly improved [4]. Therefore, finding the right treatment for AKI is essential.
Sirt1 is a mammalian NAD+-dependent protein deacetylase consisting of 747 amino acids that form a highly conserved catalytic core domain with extended highly ordered N-terminal and C-terminal regions. Its core domain is the region where NAD+-dependent deacetylation occurs [5]. Sirt1 is located in the nucleus and regulates the target in the cytoplasm by shuttle. Sirt1 can inhibit inflammation and apoptosis, and its biological function mainly depends on its deacetylase activity [6]. Recent studies have shown that Sirt1 also inhibits Toll-like receptor 4-induced transcription of NF-κB in renal medullary collecting duct cells. Acetylation of NF-κB is extremely important for its transcriptional activity, while Sirt1 inhibits the NF-κB signaling pathway, mainly by deacetylation [7].
Calcitonin gene-related peptide (CGRP) is an active peptide and plays an important role in maintaining normal cardiovascular function. Recent studies have found that CGRP also has a variety of biological functions. CGRP inhibits lymphocyte proliferation, inhibits the production of chemokines, inhibits the release of IL-2, IFN-γ, TNF-α, and other cytokines by T cells, and can prevent inflammatory damage in the liver of mice [8]. CGRP can inhibit macrophage exposure to heat-killing virus-induced lymphocyte proliferation response, inhibit NK cell-killing function, and inhibit IL-2 activation of T cells. Studies have shown that CGRP can inhibit IL-7-mediated B lymphocyte maturation and differentiation. CGRP induces Langerhans cells to present specific antigens and stimulate T cells to transform into Th2 subpopulations. Mature and immature dendritic cells express type 1 CGRP receptors on the surface. Binding to CGRP may attenuate the proliferation of mature dendritic cells by downregulating the expression of CD86 and HLA-DR. In addition, CGRP can inhibit dendritic cells and macrophages to present antigens, thereby reducing cellular immune responses [9].
Our study found that CGRP can regulate the expression of Sirt1 to play an important anti-inflammatory role. AKI is accompanied by many inflammatory reactions in the kidney, and the anti-inflammatory effects of CGRP may have a good effect on AKI. This may provide new directions for the treatment of clinical AKI.
Material and Methods
CELL CULTURE AND TREATMENT:
HK-2 cells were purchased from Shanghai Yaji Biotechnology Co. (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Rockville, MD, USA) was used to culture HK-2 cells. We prepared 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and 1% penicillin plus streptomycin (Gibco, Rockville, MD, USA) in DMEM to make complete medium. The cells were cultured in an incubator at 37°C and 5% CO2. When the cell growth density was about 60%, we added or transfected the cells. Lipopolysaccharide (LPS, Sigma, St. Louis, MO, USA) was used to induce HK-2 cell damage, while CGRP (Invitrogen, Carlsbad, CA, USA) was used to stimulate HK-2 cells to detect their effects.
CELL TRANSFECTION:
HK-2 cells were passaged to 6-well plates and after cell growth density reached approximately 60%, we transfected Lenti-NC, Lenti-Sirt1, siRNA-negative control (NC), or siRNA-Sirt1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Lenti-Sirt1 was used to increase Sirt1 in HK-2 cells, while siRNA-Sirt1 was used to reduce Sirt1 in HK-2 cells. Lenti-NC transfected cells were used as a negative control. The primer for siRNA-Sirt1 was made by Shanghai Jierui Biotech Co. (Shanghai, China), and the primer sequence was 5’-ACUUUGCUGUAACCCUGUAdTdT-3’.
ANIMALS AND GROUPING:
Specific pathogen-free (SPF) grade C57/BL6 male mice were used in the study. All mice were housed in SPF barrier facilities and given standard-grade feed and drinking water. The mice in this study were divided into 3 groups: control group, AKI group, and AKI+CGRP group. AKI models were constructed in both AKI and AKI+CGRP mice. We constructed the AKI model by intraperitoneal injection of 10 mg/kg LPS into mice, and 24 hours later, we collected urine and killed the mice [10]. In addition, the AKI+CGRP group received a daily intraperitoneal injection of CGRP 1 nmol/kg 1 week before and 1 day after LPS injection. This study was approved by the Animal Ethics Committee of The Fourth People’s Hospital of Jinan Animal Center.
ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA):
We measured the expression levels of cell supernatants, mouse urine, and serum-related indicators by ELISA. The cell supernatant, mouse urine, or serum was taken out and centrifuged to remove the precipitate, and then we used the ELISA kits (Lianke, Hangzhou, China) to detect IL-1β, TNF-α, KIM-1, or NGAL.
MALONDIALDEHYDE (MDA) AND SUPEROXIDE DISMUTASE (SOD) ACTIVITY ASSAY:
After mice were sacrificed, we took the mouse kidney tissue and ground the kidney tissue into powder on ice, which was then dissolved in phosphate-buffered saline (PBS). After removing the precipitate by centrifugation, we used MDA activity kit (Lianke, Hangzhou, China) and SOD activity kit (Lianke, Hangzhou, China) to detect the levels of MDA and SOD in PBS solution.
HEMATOXYLIN-EOSIN (HE) STAINING:
After the mice were sacrificed, we immediately removed the mouse kidney tissue and placed it in 4% paraformaldehyde solution for 24 hours. Then, we washed the kidney tissue with PBS and embedded the tissue in paraffin after dehydration. We then used a microtome to make paraffin sections and placed the paraffin sections in a 37°C incubator for 24 hours. Then, after dewaxing and hydration, we soaked the paraffin sections in hematoxylin stain for 5 minutes and rinsed the paraffin sections with running water. Then, we placed the paraffin sections in eosin solution for 5 minutes. Finally, after dehydration and sealing, we observed and recorded the staining results using a microscope.
WESTERN BLOT:
We used protein lysate to lyse cells and mouse kidney tissue and detect protein concentration by bicinchoninic acid (BCA) kit (Beyotime, Shanghai, China). Equal amounts of protein were added to each well of the electrophoresis gel for sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). We then transferred the protein to the polyvinylidene fluoride (PVDF) membranes (Roche, Basel, Switzerland) by transfer. After washing the PVDF membranes with phosphate-buffered saline-tween (PBST), we incubated the PVDF membranes with 5% BSA-PBST for 1 hour. We then incubated the PVDF membrane at 4°C overnight with primary antibody dilution (KIM-1, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA; NGAL, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA; IL-6, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA; IL-8, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA; Sirt1, 1: 3000, Rabbit, Abcam, Cambridge, MA, USA; β-actin, 1: 5000, Rabbit, Abcam, Cambridge, MA, USA). After washing the PVDF membranes the next day, we used secondary antibody dilution (Goat anti-rabbit, 1: 3000, Abcam, Cambridge, MA, USA) to incubate the PVDF membrane for 1 hour. Finally, we observed the protein bands by chemiluminescence and analyzed them.
RNA ISOLATION AND QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION (RT-PCR):
We used TRIzol (Invitrogen, Carlsbad, CA, USA) to solubilize HK-2 cells or mouse kidney tissue and extract total RNA. RNA was reversed to complementary deoxyribose nucleic acid (cDNA) by reverse transcription. We then amplified the corresponding cDNA fragments with different primers using the SYBR Green kit (Beyotime, Shanghai, China). We used the expression level of endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as control to compare the expression levels of other cDNA fragments and represent them using 2−CT. Primer sequences for RT-PCR were as follows:
CELL COUNTING KIT-8 (CCK8) ASSAY:
We cultured HK-2 cells and passaged them into 96-well plates. After the cell growth density reached about 60%, we stimulated HK-2 cells for 24 hours with different concentrations of CGRP and LPS. We then added 10 μL of CCK8 reagent (Dojindo, Kumamoto, Japan) to each well and continued incubation for 2 hours. Then, we used a microplate reader to measure the absorbance of each well.
IMMUNOCYTOFLUORESCENCE (IF) STAINING:
We used 24-well plates to culture HK-2 cells and added cell slides to the culture dishes. After treating the cells, we took out the culture dish and discarded the medium. We then fixed the cells with 4% paraformaldehyde for 10 minutes and continued incubation with 0.2% Triton-PBS for 15 minutes. After washing the cells with PBS, we blocked the cells with 10% goat serum for 30 minutes. Then, we discarded the liquid in the culture dish and added the primary antibody dilution (KIM-1, 1: 500, rabbit, Abcam, Cambridge, MA, USA; NGAL, 1: 500, rabbit, Abcam, Cambridge, MA, USA; Sirt1, 1: 500, rabbit, Abcam, Cambridge, MA, USA). The cells were incubated overnight at 4°C. The next day, after washing the cells with PBS, we incubated the cells with fluorescent secondary antibody dilution (Goat anti-rabbit-FITC, 1: 500, Abcam, Cambridge, MA, USA) for 1 hour and washed the cells. Then, we fixed the cells to the slides using a 4’,6-diamidino-2-phenylindole (DAPI)-containing capsule and observed the staining results using a fluorescence microscope.
STATISTICAL ANALYSIS:
We used Statistical Product and Service Solutions (SPSS) 21.0 (IBM, Armonk, NY, USA) and STATA 15.0 software (London, UK) to analyze the data from this study. Data were represented as mean±SD (Standard Deviation). Comparison between multiple groups was done using One-way ANOVA test followed by the least significant difference post hoc test.
Results
CGRP ATTENUATES LPS-INDUCED DAMAGE TO HK-2 CELLS:
To determine whether CGRP can protect HK-2 cells, we used LPS to induce HK-2 cell damage and stimulated HK-2 cells with CGRP. The results of CCK8 showed that CGRP can increase HK-2 cell activity and 10 nmol/L CGRP is the optimal concentration (Figure 1A), while 1 μg/mL LPS can significantly reduce HK-2 cell activity (Figure 1B). The results of ELISA showed that LPS-induced inflammatory factors (IL-1β and TNF-α) in HK-2 cells were significantly increased and CGRP was able to reduce the expression of inflammatory factors (IL-1β and TNF-α) (Figure 1C, 1D). In addition, we examined the expression of renal injury biomarkers KIM-1 and NGAL by IF staining and results showed that CGRP can significantly reduce KIM-1 and NGAL (Figure 1E, 1F). The results of Western blot (Figure 1G) and RT-PCR (Figure 1H) also showed that CGRP reduced KIM-1, BGAL, IL-6, and IL-8 levels.
CGRP INCREASED THE EXPRESSION OF SIRT1 IN HK-2 CELLS:
To determine the mechanism of CGRP, we examined Sirt1 in HK-2 cells. The results of IF staining (Figure 2A) showed that Sirt1 was decreased in LPS-induced HK-2 cells, indicating that the damage of HK-2 cells was accompanied by the decrease of Sirt1. However, in CGRP-stimulated HK-2 cells, the expression of Sirt1 increased. The results of Western blot (Figure 2B) and RT-PCR (Figure 2C) were similar to those of IF staining.
OVEREXPRESSION OF SIRT1 REDUCED DAMAGE AND INFLAMMATION OF HK-2 CELLS:
To determine the relationship between Sirt1 and HK-2 cell damage, we constructed HK-2 cells overexpressing Sirt1 by lentiviral transfection. The results of Western blot (Figure 3A) and RT-PCR (Figure 3B) confirmed the transfection efficiency of Lenti-Sirt1. The results of IF staining (Figure 3C, 3D) showed that the expression of KIM-1 and NGAL in HK-2 cells was significantly decreased after Sirt1 overexpression and Sirt1 decreased the expression of KIM-1 and NGAL, indicating that Sirt1 can reduce LPS-induced HK-2 cells damage. The results of ELISA (Figure 3E, 3F) and RT-PCR (Figure 3G) showed that Sirt1 significantly reduced inflammatory factors (IL-1β and TNF-α) in HK-2 cells. These results indicated that overexpression of Sirt1 could protect HK-2 cells.
SILENCE OF SIRT1 ATTENUATED THE PROTECTIVE EFFECT OF CGRP ON HK-2 CELLS:
We verified whether CGRP protects HK-2 cells by modulating Sirt1. The results of Western blot (Figure 4A) and RT-PCR (Figure 4B) confirmed the transfection efficiency of siRNA-Sirt1. The results of IF staining (Figure 4C, 4D) showed that the expression of KIM-1 and NGAL was significantly increased after silencing the expression of Sirt1, indicating that silencing of Sirt1 aggravated the damage to HK-2 cells. The results of ELISA (Figure 4E, 4F) and RT-PCR (Figure 4G) showed that silence of Sirt1 also aggravated the inflammation of HK-2 cells, which was manifested by an increase in IL-1β and TNF-α. These results indicated that the therapeutic effect of CGRP on HK-2 cells was inhibited after reducing Sirt1 in HK-2 cells.
EXOGENOUS CGRP ATTENUATED LPS-INDUCED MOUSE AKI:
To verify the effect of CGRP on AKI, we used LPS to induce mouse AKI and pretreated mice with CGRP. The results of HE staining (Figure 5A) showed that the kidney tissues in the AKI group were swollen and there was inflammatory cell infiltration in the interstitium. The morphology of the kidney tissue of the mice in the AKI+CGRP group was significantly improved. In addition, CGRP also significantly reduced the expression of MDA in mouse kidney tissue and increased the expression of SOD, indicating that CGRP improved oxidative stress in kidney tissue (Figure 5B, 5C). The results of ELISA (Figure 5D–5G) showed that CGRP reduced the expression of KIM-1 and NGAL in mouse urine and decreased the expression of IL-1β and TNF-α in mouse serum. The results of Western blot (Figure 5H) and RT-PCR (Figure 5I) showed that exogenous CGRP increased the expression of Sirt1 in mouse kidney tissue, which also confirmed that CGRP can attenuate AKI by regulating Sirt1.
Discussion
Sepsis is a systemic inflammatory response caused by infection. Pathogen invasion into the body leads to tissue damage and release of dangerous signals, including the pathogen-associated molecular pattern (PAMP) of the infection itself and the damage-associated molecular pattern (DAMP) of the damaged tissue. Immune cells, endothelial cells, and renal tubular epithelial cells recognize PAMP and DAMP through pattern receptors, initiate natural immune inflammatory responses, and release a large number of inflammatory factors, resulting in sustained activation of immune system and release of pro-inflammatory factors and reactive oxygen species [11]. The kidney is one of the target organs of inflammatory cytokine attack and is highly susceptible to damage. Tubular capillaries are similar to tubular epithelial cells, and activated white blood cells leaving tubular capillaries can produce pro-inflammatory factors and DAMP [12]. The accumulation of inflammatory cells increases the pressure between the tubules and the stroma, leading to renal microcirculatory disorders and tubule occlusion. Therefore, we used LPS to induce sepsis in mice to construct a mouse AKI model. The results showed that LPS-induced renal tubular epithelial cells in mice showed apoptosis and inflammatory cell infiltration in the stroma. In addition, LPS-induced HK-2 cells also showed significant inflammatory response [13]. SOD is a metal enzyme widely existing in the body, and is a good superoxide anion free radical scavenger. SOD can catalyze the superoxide anion free radicals in the cells to generate oxygen and hydrogen peroxide [14]. MDA is a product of lipid peroxidation. It is not only a biomarker of oxidative stress, but also has certain toxic and adverse effects [15]. Therefore, we also examined the changes of MDA and SOD activity in mice, and found that CGRP can reduce the MDA activity and increase the SOD activity. This showed that CGRP can reduce the level of oxidative stress in mice. NGAL is the most widely studied biomarker for predicting AKI and its prognosis. NGAL synthesis increased and accumulated in human renal tubules, blood and urine, which was the earliest biomarker for the elevation of AKI after the occurrence of AKI. Some authors called it “troponin of kidney” [16]. KIM-1 is a transmembrane protein that is significantly increased in patients with ischemic or toxic AKI, and is used in combination with NGAL to diagnose AKI [17]. The inhibitory effect of CGRP on kIM-1 and NGAL in HK-2 cells and mice demonstrated its renal-protective effect.
The Sirtuins family of proteins is an ortholog of mammalian yeast silent information regulator 2 (Sir2). The Sirtuins family consists of 7 members, Sirt1 to Sirt7, of which Sirt1 has the highest homology with yeast Sir2 and is the most widely studied member. Although Sir2 was originally thought to be a histone deacetylase, Sirt1 has histone and non-histone substrates [18]. The endogenous substrate of Sirt1 is very rich, including p53, NF-κB, and c-Jun. Each of its deacetylation reactions hydrolyzes a NAD+, and thus Sirt1 is also known as NAD+-dependent protein deacetylase. Sirt1’s deacetylase activity confers multiple immunomodulatory functions and is involved in the development of partial immune diseases [19]. Sirt1 is directly in contact with the NF-κB subunit ReIA/p56, causing its lys310 terminal to deacetylate, while the lys310 terminal is an important target for NF-κB transcriptional activity. It was found that smoking-mediated release of monocyte and rat lung pro-inflammatory factors is regulated by the action of Sirt1 and NF-κB [20]. Sirt1 inhibitors Sirtinol and Sirt1 knockdown have an increased effect on the release of inflammatory factors, while Sirt1 agonist SRT 1720 inhibits the release of smoking-mediated pro-inflammatory factors. In addition, Sirt1 levels and activity were decreased in patients with COPD [21]. Downregulation of Sirt1 causes an increase in lung pro-inflammatory factor via the NF-κB pathway. Recent studies have shown that the deacetylation of Sirt1 can cause downregulation of cyclooxygenase-2 gene expression, suggesting the multi-target characteristics of Sirt1 in inflammatory regulation [22]. In our study, LPS-induced expression of Sirt1 in HK-2 cells and mouse kidneys was reduced. However, after treatment of HK-2 cells and mice with CGRP, the inflammation levels in HK-2 cells and mouse kidneys were reduced, accompanied by an increase in Sirt1 levels. Overexpression of Sirt1 in HK-2 cells also had protective effects on HK-2 cells, suggesting that the protective effect of CGRP on HK-2 cells may be related to the regulation of Sirt1. In addition, the reduction of the therapeutic effect of CGRP on HK-2 cells after reducing the expression of Sirt1 in HK-2 cells also confirmed that the alleviation of AKI by CGRP may be related to the regulation of Sirt1.
Wang et al. [23] proposed that CGRP is one of the mediators of the neuro-immune system. Studies have shown that when cerebral hemorrhage and hemorrhagic, endotoxin, septic shock, the release of CGRP significantly increased. Some common inflammatory mediators (such as prostaglandins, bradykinin) and environmental factors such as pH, high lactic acid, and high-tension liquid can directly stimulate the nerve excitation containing CGRP, causing an increase in the release of CGRP. Increased levels of lactic acid can enhance the release of CGRP caused by the above inflammatory mediators [24]. Studies have confirmed that CGRP regulates a variety of immune functions; for example, CGRP regulates multiple functions of endotoxin-activated macrophages and has the function of dual regulation of IL-6 production (a small dose increases and a high dose inhibits IL-6) [25]. Therefore, CGRP has promising prospects due to its superior anti-inflammatory effects. We believe that this study can help make a new breakthrough in the prevention and treatment of AKI.
Conclusions
CGRP and overexpression of Sirt1 can effectively alleviate the damage of HK-2 cells and reduce their inflammation. The expression of Sirt1 in HK-2 cells stimulated by CGRP was significantly increased and the inhibition of Sirt1 attenuated the protective effect of CGRP on HK-2 cells. Therefore, CGRP has a good protective effect on LPS-induced HK-2 cells.
Figures
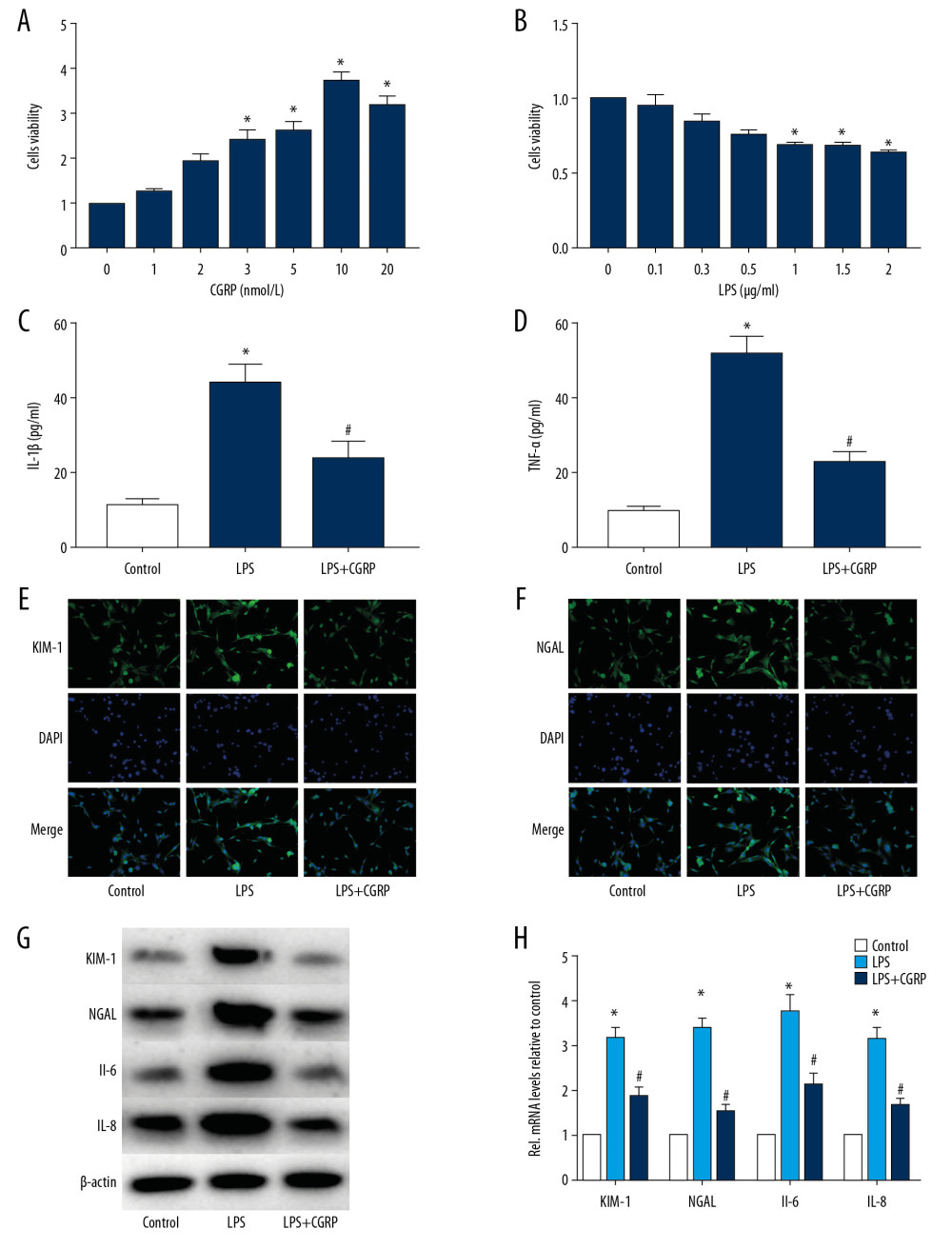 Figure 1. CGRP attenuates LPS-induced damage to HK-2 cells. (A) CCK8 assay of CGRP; (B) CCK8 assay of LPS; (C, D) ELISA results of IL-1β and TNF-α; (E, F) IF staining results of KIM-1 and NGAL (magnification×400); (G, H) Western blot and RT-PCR results of KIM-1, NGAL, IL-6 and IL-8. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group).
Figure 1. CGRP attenuates LPS-induced damage to HK-2 cells. (A) CCK8 assay of CGRP; (B) CCK8 assay of LPS; (C, D) ELISA results of IL-1β and TNF-α; (E, F) IF staining results of KIM-1 and NGAL (magnification×400); (G, H) Western blot and RT-PCR results of KIM-1, NGAL, IL-6 and IL-8. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group). 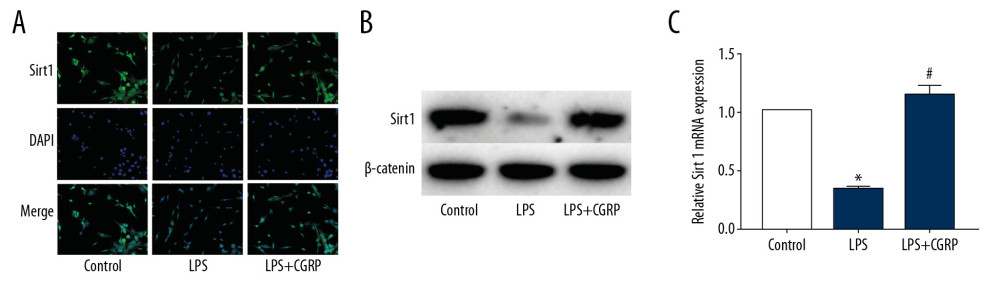 Figure 2. CGRP increases the expression of Sirt1 in HK-2 cells. (A) IF staining result of Sirt1 (magnification ×400); (B) Western blot result of Sirt1; (C) RT-PCR result of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group).
Figure 2. CGRP increases the expression of Sirt1 in HK-2 cells. (A) IF staining result of Sirt1 (magnification ×400); (B) Western blot result of Sirt1; (C) RT-PCR result of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group). 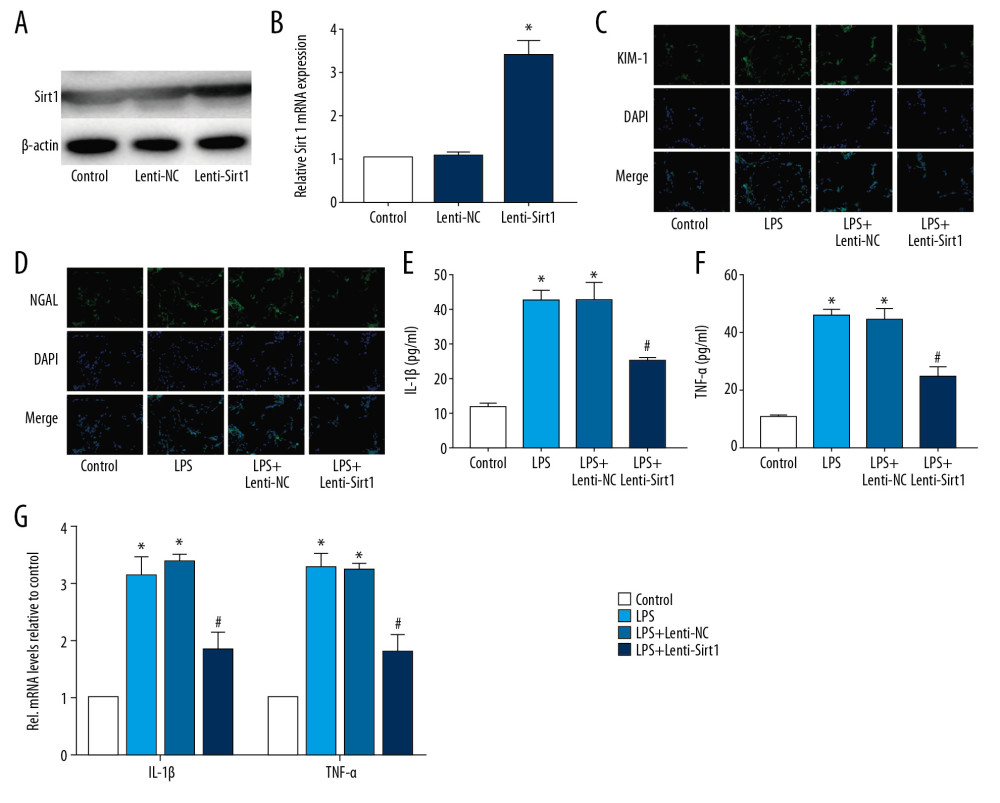 Figure 3. Overexpression of Sirt1 reduces damage and inflammation of HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining results of KIM-1 and NGAL (magnification×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS+Lenti-NC group).
Figure 3. Overexpression of Sirt1 reduces damage and inflammation of HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining results of KIM-1 and NGAL (magnification×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS+Lenti-NC group). 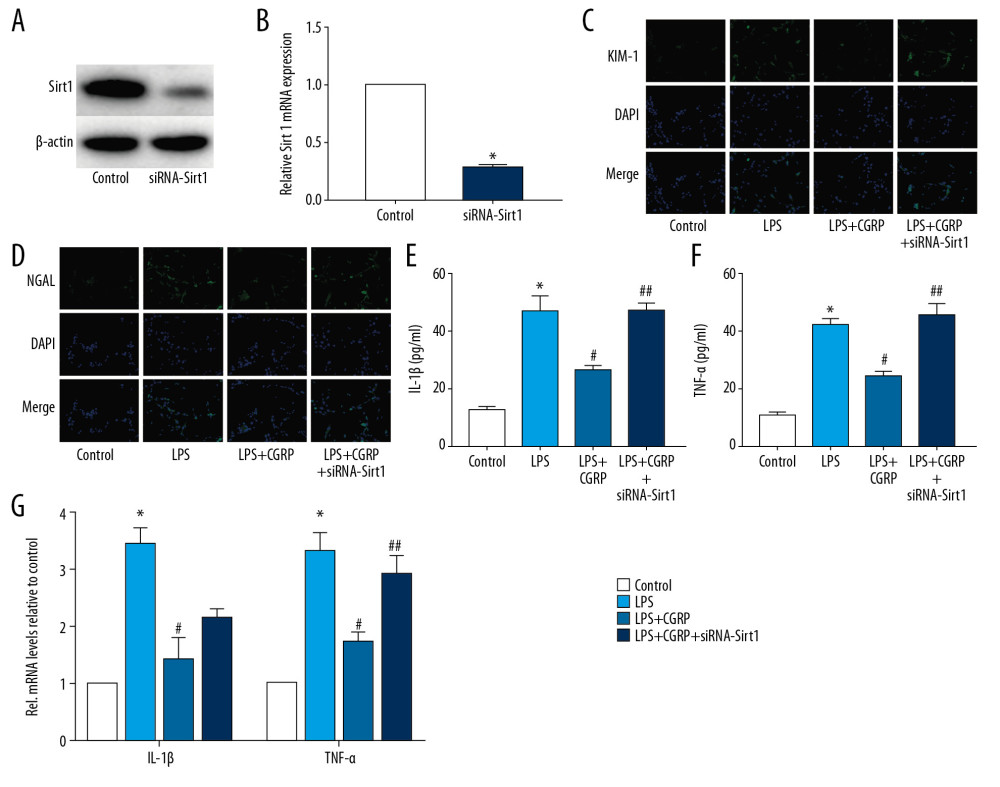 Figure 4. Silence of Sirt1 attenuates the protective effect of CGRP on HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining of KIM-1 and NGAL (magnification ×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group, # P<0.05 vs. the LPS group and ## P<0.05 vs. the LPS+CGRP group).
Figure 4. Silence of Sirt1 attenuates the protective effect of CGRP on HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining of KIM-1 and NGAL (magnification ×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group, # P<0.05 vs. the LPS group and ## P<0.05 vs. the LPS+CGRP group). 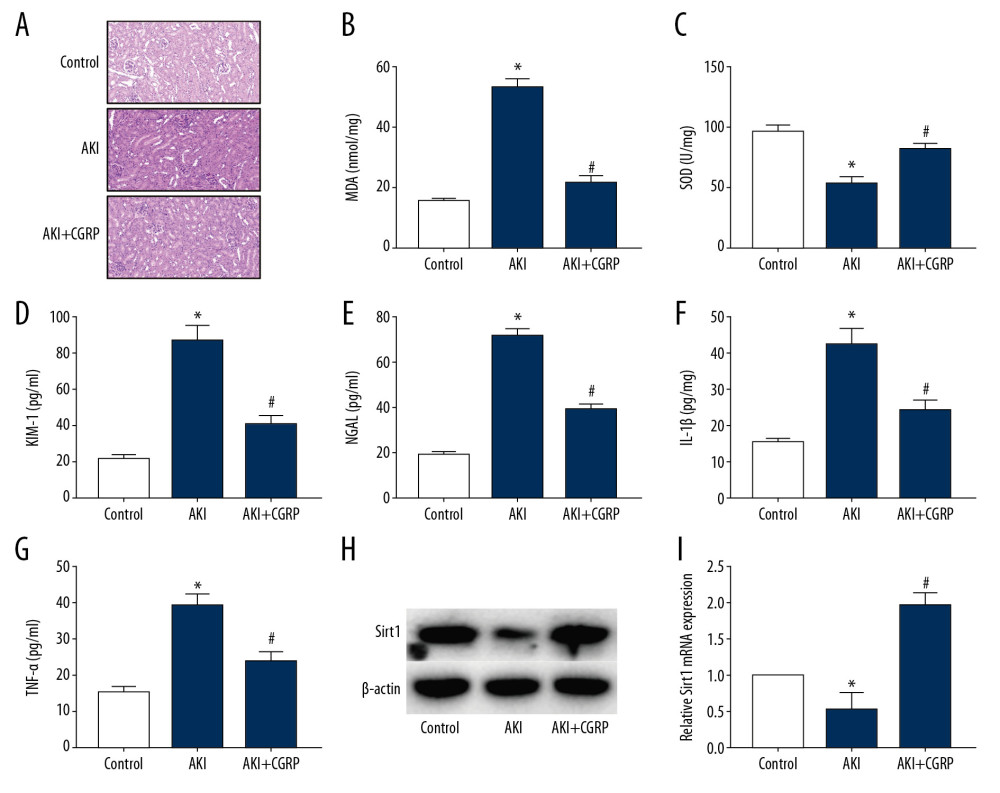 Figure 5. Exogenous CGRP attenuates LPS-induced mouse AKI. (A) HE staining results of mice kidney (magnification ×400); (B) MDA activity in mice kidney; (C) SOD activity in mice kidney; (D–G) ELISA results of KIM-1, NGAL, IL-1β and TNF-α. (H, I) Western blot and RT-PCR results of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the AKI group).
Figure 5. Exogenous CGRP attenuates LPS-induced mouse AKI. (A) HE staining results of mice kidney (magnification ×400); (B) MDA activity in mice kidney; (C) SOD activity in mice kidney; (D–G) ELISA results of KIM-1, NGAL, IL-1β and TNF-α. (H, I) Western blot and RT-PCR results of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the AKI group). References
1. Zhang J, Wang CJ, Tang XM, Wei YK, Urinary miR-26b as a potential biomarker for patients with sepsis-associated acute kidney injury: A Chinese population-based study: Eur Rev Med Pharmacol Sci, 2018; 22; 4604-10
2. Sykes L, Nipah R, Kalra P, Green D, A narrative review of the impact of interventions in acute kidney injury: J Nephrol, 2018; 31; 523-35
3. Hertzberg D, Ryden L, Sartipy U, Holzmann MImportant to investigate the cause of acute kidney injury. Treatment should aim to limit the damage and prevent progression: Lakartidningen, 2016; 113 DWDH [in Swedish]
4. Burmeister DM, Gomez BI, Dubick MA, Molecular mechanisms of trauma-induced acute kidney injury: Inflammatory and metabolic insights from animal models: Biochim Biophys Acta Mol Basis Dis, 2017; 1863; 2661-71
5. Tang BL, Sirt1 and the mitochondria: Mol Cells, 2016; 39; 87-95
6. Imperatore F, Maurizio J, Vargas AS, SIRT1 regulates macrophage self-renewal: EMBO J, 2017; 36; 2353-72
7. Meng X, Tan J, Li M, Sirt1: Role under the condition of ischemia/hypoxia: Cell Mol Neurobiol, 2017; 37; 17-28
8. Russell FA, King R, Smillie SJ, Calcitonin gene-related peptide: Physiology and pathophysiology: Physiol Rev, 2014; 94; 1099-142
9. Tepper DE, Calcitonin gene-related peptide targeted therapy for migraine: Headache, 2016; 56; 447-48
10. Jin W, Zhao Y, Hu Y, Stromal cell-derived factor-1 enhances the therapeutic effects of human endometrial regenerative cells in a mouse sepsis model: Stem Cells Int, 2020; 2020 4820543
11. Rello J, Valenzuela-Sanchez F, Ruiz-Rodriguez M, Moyano S, Sepsis: A review of advances in management: Adv Ther, 2017; 34; 2393-411
12. Faix JD, Biomarkers of sepsis: Crit Rev Clin Lab Sci, 2013; 50; 23-36
13. Ludwig KR, Hummon AB, Mass spectrometry for the discovery of biomarkers of sepsis: Mol Biosyst, 2017; 13; 648-64
14. Mansuroğlu B, Derman S, Yaba A, Kızılbey K, Protective effect of chemically modified SOD on lipid peroxidation and antioxidant status in diabetic rats: Int J Biol Macromol, 2015; 72; 79-87
15. Dillioglugil MO, Kir HM, Demir C, Effect of pentylenetetrazole and sound stimulation induced single and repeated convulsive seizures on the MDA, GSH and NO levels, and SOD activities in rat liver and kidney tissues: Brain Res Bull, 2010; 83(6); 356-59
16. Wang E, Chiou YY, Jeng WY, Overexpression of exogenous kidney-specific Ngal attenuates progressive cyst development and prolongs lifespan in a murine model of polycystic kidney disease: Kidney Int, 2017; 91(2); 412-22
17. Tanase DM, Gosav EM, Radu S, The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity: Int J Mol Sci, 2019; 20(20); 5238
18. Liu L, Liu C, Zhang Q, SIRT1-mediated transcriptional regulation of SOX2 is important for self-renewal of liver cancer stem cells: Hepatology, 2016; 64; 814-27
19. Ren Y, Du C, Shi Y, The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress: Int J Mol Med, 2017; 39; 1317-24
20. Clark-Knowles KV, He X, Jardine K, Reversible modulation of SIRT1 activity in a mouse strain: PLoS One, 2017; 12; e173002
21. Taka C, Hayashi R, Shimokawa K, SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients: Int J Chron Obstruct Pulmon Dis, 2017; 12; 3237-44
22. Jang J, Huh YJ, Cho HJ, SIRT1 enhances the survival of human embryonic stem cells by promoting DNA repair: Stem Cell Rep, 2017; 9; 629-41
23. Assas BM, Pennock JI, Miyan JA, Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis: Front Neurosci, 2014; 8; 23
24. Guo Z, Liu N, Chen L, Independent roles of CGRP in cardioprotection and hemodynamic regulation in ischemic postconditioning: Eur J Pharmacol, 2018; 828; 18-25
25. Glowka TR, Steinebach A, Stein K, The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus: Neurogastroenterol Motil, 2015; 27; 1038-49
Figures
 Figure 1. CGRP attenuates LPS-induced damage to HK-2 cells. (A) CCK8 assay of CGRP; (B) CCK8 assay of LPS; (C, D) ELISA results of IL-1β and TNF-α; (E, F) IF staining results of KIM-1 and NGAL (magnification×400); (G, H) Western blot and RT-PCR results of KIM-1, NGAL, IL-6 and IL-8. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group).
Figure 1. CGRP attenuates LPS-induced damage to HK-2 cells. (A) CCK8 assay of CGRP; (B) CCK8 assay of LPS; (C, D) ELISA results of IL-1β and TNF-α; (E, F) IF staining results of KIM-1 and NGAL (magnification×400); (G, H) Western blot and RT-PCR results of KIM-1, NGAL, IL-6 and IL-8. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group). Figure 2. CGRP increases the expression of Sirt1 in HK-2 cells. (A) IF staining result of Sirt1 (magnification ×400); (B) Western blot result of Sirt1; (C) RT-PCR result of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group).
Figure 2. CGRP increases the expression of Sirt1 in HK-2 cells. (A) IF staining result of Sirt1 (magnification ×400); (B) Western blot result of Sirt1; (C) RT-PCR result of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS group). Figure 3. Overexpression of Sirt1 reduces damage and inflammation of HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining results of KIM-1 and NGAL (magnification×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS+Lenti-NC group).
Figure 3. Overexpression of Sirt1 reduces damage and inflammation of HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining results of KIM-1 and NGAL (magnification×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group and # P<0.05 vs. the LPS+Lenti-NC group). Figure 4. Silence of Sirt1 attenuates the protective effect of CGRP on HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining of KIM-1 and NGAL (magnification ×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group, # P<0.05 vs. the LPS group and ## P<0.05 vs. the LPS+CGRP group).
Figure 4. Silence of Sirt1 attenuates the protective effect of CGRP on HK-2 cells. (A, B) Western blot and RT-PCR results of Sirt1; (C, D) IF staining of KIM-1 and NGAL (magnification ×400); (E, F) ELISA results of IL-1β and TNF-α; (G) RT-PCR results of IL-1β and TNF-α. (* P<0.05 vs. the control group, # P<0.05 vs. the LPS group and ## P<0.05 vs. the LPS+CGRP group). Figure 5. Exogenous CGRP attenuates LPS-induced mouse AKI. (A) HE staining results of mice kidney (magnification ×400); (B) MDA activity in mice kidney; (C) SOD activity in mice kidney; (D–G) ELISA results of KIM-1, NGAL, IL-1β and TNF-α. (H, I) Western blot and RT-PCR results of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the AKI group).
Figure 5. Exogenous CGRP attenuates LPS-induced mouse AKI. (A) HE staining results of mice kidney (magnification ×400); (B) MDA activity in mice kidney; (C) SOD activity in mice kidney; (D–G) ELISA results of KIM-1, NGAL, IL-1β and TNF-α. (H, I) Western blot and RT-PCR results of Sirt1. (* P<0.05 vs. the control group and # P<0.05 vs. the AKI group). In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








