12 August 2020: Clinical Research
Effect of 2 Different Dosages of Rosuvastatin on Prognosis of Acute Myocardial Infarction Patients with New-Onset Atrial Fibrillation in Jinan, China
Jie Zhang12ABCDEFG, Ruiqi Feng3DEF, Misbahul Ferdous45DEF, Bo Dong5BCDG, Haitao Yuan5ABCDF, Peng Zhao5ABCDEFG*DOI: 10.12659/MSM.925666
Med Sci Monit 2020; 26:e925666
Abstract
BACKGROUND: Atrial fibrillation (AF) often occurs in patients with acute myocardial infarction (AMI). This study aimed to observe the influence of different dosages of rosuvastatin on the prognosis of AMI patients with AF.
MATERIAL AND METHODS: We performed an observational, retrospective cohort study in Jinan, China, in which 323 AMI patients were recruited. All patients were randomized to receive optimal medication treatment and 10 mg or 20 mg of rosuvastatin. Holter monitor results, serum lipid levels, and heart function were recorded. We used multivariate Cox and Kaplan-Meier analyses to assess the independent factors and differences in AF and ischemia events and safety of rosuvastatin administered at different dosages.
RESULTS: TC, LDL-C, and TG at 1 and 12 months were significantly lower compared with those observed prior to treatment in both groups. The heart function of both groups was significantly improved after 12 months of treatment, especially in the 20 mg group. Multivariate Cox analysis showed that different dosages of rosuvastatin, age, smoking, drinking alcohol, and diabetes are independent factors related to the occurrence of AF and ischemic events. In addition, according to Kaplan-Meier analysis, no significant difference in adverse clinical events existed at different dosages of rosuvastatin.
CONCLUSIONS: Treatment with rosuvastatin can reduce the serum lipid level and improve cardiac function. Different dosages of rosuvastatin, age, smoking, drinking alcohol, and diabetes are independent risk factors for AF and ischemia events. The results suggested it is safe to use 20 mg rosuvastatin in the 12 months after hospital admission.
Keywords: Atrial Fibrillation, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Dose-Response Relationship, Drug, rosuvastatin calcium
Background
Acute myocardial infarction (AMI) is acute myocardial necrosis caused by persistent and severe myocardial ischemia. Clinical manifestations include persistent severe pain, acute circulatory dysfunction, and arrhythmia. Patients with acute coronary syndrome (ACS) may develop new-onset atrial fibrillation (AF). About 10–20% of patients with AMI have AF [1,2]. New-onset AF often occurs in patients with myocardial infarction and the incidence of AF will increase the rate of complications and mortality and the risk of AF at follow-up [3–5]. AF is more likely to occur in older patients with AMI and in those with higher Killip class or left ventricular dysfunction [6]. The factors affecting AF onset in the acute phase of MI are left ventricular dysfunction, notably Killip class IV and high heart rate (>100 bpm) at admission [7]. AF is a common clinical arrhythmia. Long-term restoration and maintenance of sinus rhythm using the currently available antiarrhythmic agents is a clinical challenge.
The 2018 AHA/ACC Guidelines on Management of Blood Cholesterol confirmed the relationship between low-density lipoprotein cholesterol (LDL-C) and atherosclerotic cardiovascular disease (ASCVD) [8]. The 2019 ESC/EAS Guidelines for the Management of Dyslipidemias recommend the new goal of reaching a 50% LDL-C reduction from baseline and/or <1.4 mmol/L (<55 mg/dL) [9]. All ACS patients with no contraindications should be given high-intensity statin therapy, regardless of initial LDL-C values. Statin therapy is recommended as the first-line treatment for primary prevention of ASCVD [10]. The HOPE-3 trial showed the importance of statins in the treatment of atherosclerosis and as secondary prevention in acute myocardial infarction [11]. Statin pretreatment can effectively improve myocardial perfusion in patients with STEMI, can improve the prognosis of coronary heart disease [12], and possibly prevent AF by significantly lowing C-reactive protein levels [13]. Research suggests that statins may potentially prevent or deter AF, irrespective of their effects on lipids, by anti-inflammatory action. Other beneficial effects of statin therapy are seen in controlling inflammation, oxidation, and thrombosis [14–16]. With more in-depth analysis of the pleiotropic effects of statin in coronary heart disease, the role of statin, in addition to its ability to control lipid level, has also become the focus of basic and clinical researchers. A meta-analysis showed the benefits of statin use, which can prevent AF [17]. High-dose rosuvastatin treatment can reduce the incidence of contrast-induced nephropathy in patients with PCI [18]. Another meta-analysis showed that high-dose rosuvastatin can significantly reduce hs-CRP, LDL-C, and cTnT levels in ACS patients with PCI in China [19]. Although studies showed the benefit of statins in reducing AF occurrence in ACS patients, there is insufficient data on the role of rosuvastatin in reducing new-onset AF in AMI patients. The purpose of the present study was to investigate the effect of 2 dosages of rosuvastatin on the incidence of AF and ischemic events in AMI patients. We also observed the safety of using different dosages of rosuvastatin to determine the optimal dosage of rosuvastatin.
Material and Methods
DATA SOURCES AND STUDY POPULATION:
This was an observational, retrospective cohort study performed in the central and east districts of Shandong Provincial Hospital affiliated to Shandong First Medical University in Jinan, China. The reviewed period was from January 2014 to June 2018. The data were obtained from the medical records database in the Medical Records Department. Using the key words “acute myocardial infarction” and “rosuvastatin”, we found 376 cases. The patients were offered optimal medication treatment and 10 mg or 20 mg rosuvastatin. Clinical follow-up by telephone interview, outpatient clinic visit, or at readmission were conducted until the end of the 2019. We recruited 323 patients whose data were comprehensive and complete according to the inclusion criteria. Among them, 175 were offered optimal medication treatment and 10 mg of rosuvastatin, while 148 patients were offered optimal medication treatment and 20 mg of rosuvastatin. There were 236 males and 87 females with an age range of 33–83 years. The main exclusion criteria were: (1) history of AF, (2) hyperthyroidism, (3) moderate-severe rheumatic valvular disease, (4) infectious diseases, immune system diseases, and sepsis, (5) recent use of anti-inflammatory drugs and antioxidant therapy, (6) combined liver and kidney dysfunction and other serious systemic diseases, (7) combined hypertension and diabetes mellitus not treated effectively, (8) other acute or chronic diseases of the blood and cancer, and (9) psychotic. The study was designed by all the authors and was approved by the Ethics Committees at Shandong Provincial Hospital affiliated to Shandong First Medical University. Consent was exempted due to retrospective enrollment.
TREATMENT:
All patients were treated according to the 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction [20,21]. Optimal treatment included antiplatelet, anticoagulant drugs, nitrates, ACEI/ARB, beta blockers, thrombolysis, or/and PCI treatment. Based on the above treatment, the patients were given rosuvastatin (Crestor, Astra Zeneca, USA) treatment once daily after admission. The patients were randomly allocated to receive rosuvastatin 10 mg or 20 mg treatment. A 12-lead electrocardiogram (ECG) was recorded at admission in all patients. Each patient underwent an electrocardiogram at each visit during the study. AF was considered to be rapid oscillations or fibrillatory waves, which vary in size, shape, and time, and are accompanied by irregular ventricular responses. Follow-up was routinely performed by telephone interview or at an outpatient clinic at 1, 3, 6 and 12 months after discharge. During the 12-month follow-up, 24-h Holter ECG monitoring was recorded as needed. AF occurrence during the study was defined as follows: the presence of AF in the ECG performed during each planned clinical visit, AF as a cause of heart dysfunction and/or hospital admission, and AF occurrence during hospitalization. Anticoagulant agents were given to patients if AF occurred during hospitalization or follow-up. Antiarrhythmic drugs were used to reverse sinus rhythm. Blood biochemical variables were closely monitored during medication. Patients stopped taking rosuvastatin if any of the following events occur: myalgia, creatine kinase (CK) more than 5 times the upper limit of normal, alanine aminotransferase (ALT) more than 3 times the normal high limit, and creatinine (Cr) above 221mmol/L. Patients who had chest pain, palpitations, and other symptoms were required to undergo ECG examinations and were admitted to the hospital if needed. The flow diagram of the main procedures is illustrated in Figure 1.
VARIABLES:
Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), creatine kinase (CK), and creatinine (Cr) levels were measured by automatic biochemistry analyzer (AU5800, Beckman Coulter, USA) in the clinical laboratory diagnostic center of Shandong Provincial Hospital affiliated to Shandong First Medical University in Jinan, China. The levels of HDL-C, LDL-C, and CK were measured by HDL-C, LDL-C, and CK Assay Kits, respectively (Directed Method and Phosphocreatine Substrate Method, Weigao Biochemistry, China). The levels of TC and TG were measured by TC and TG Assay Kits, respectively (Enzyme Method and GPO-POD Method, Beckman Coulter, USA). The levels of ALT were measured by ALT Assay Kit (LDH Method, Beckman Coulter, USA). The levels of Cr were measured by Creatinine Assay Kit (Sarcosine oxidase Method, Maccura, China). The main testing procedures were performed following the manufacturer’s instructions, including reagent preparation, parameter setting, instrument calibration, operation, quality control, and calculation. We recorded occurrences of paroxysmal, persistent, and permanent atrial fibrillation and ischemic events. Ischemic-related events include heart failure, recurrent hospitalization of coronary heart disease, sudden cardiac death, and ischemic stroke.
ECHOCARDIOGRAPHY:
All studies were performed with echocardiography equipment (Sonos 5500, Philips Medical Systems, Bothell, WA, USA) during admission and 12 months after the use of the drug. Left ventricular end-diastolic diameters (LVDd) was obtained by M-mode echocardiography from the parasternal long axis view, as recommended by the American Society of Echocardiography. The ejection fraction (EF), fractional shortening of the left ventricle (FS), and cardiac output (CO) were calculated by a modified Simpson’s method. Data from 3–5 consecutive cardiac cycles were used for analysis [22,23].
STATISTICAL ANALYSIS:
Data are presented as mean±standard deviation and were analyzed by using GraphPad Prism 8.0 and SPSS20.0 statistical analysis software. Each set of data related to 1 drug dosage was compared separately using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons or the
Results
BASELINE CHARACTERISTICS OF PATIENTS:
The average age of the study population was 62.3±9.8 years, and 77.1% of patients were men. The differences of diagnosis, age, sex, risk factors, medical history, current or recent medication, and treatment between the 2 groups were not statistically significant. Nevertheless, there were more patients with hypertension in the 10-mg group than in the 20-mg group. Diabetes was the most frequent comorbidity, but there was no statistically significant difference between the 2 groups. More than half of the patients received a β-blocker to control heart rate. After admission, 85.4% of patients were treated with PCI (Table 1).
RESULTS OF BLOOD LIPID OF PATIENTS:
After treatment with rosuvastatin for 1 and 12 months after discharge, the levels of TC, LDL-C, and TG were significantly lower than those at admission in both the 10-mg and 20-mg groups (P<0.05). No significant change was found in HDL-C after 1 or 12 months of rosuvastatin treatment (P>0.05). Compared with the 10-mg group, LDL-C was significantly lower in the 20-mg group at 1 and 12 months (P<0.05). The LDL-C level was around 1.8 mmol/L at 1 and 12 months in the 20-mg group. The results indicated that rosuvastatin effectively lowered lipid levels, and the effect of 20-mg rosuvastatin being better than that of 10-mg rosuvastatin (Figure 2).
RESULTS OF ECHOCARDIOGRAPHY OF PATIENTS:
Two groups of patients were treated with different dosages of rosuvastatin for 12 months. HR of both groups were significantly lower than before treatment (P<0.05). While there was no significant difference in LVDd of both groups post-treatment, the 20-mg group showed more improvement in left ventricular function. The LVEF, FS, and CO of the 20-mg group showed significant improvement compared to before treatment (P<0.05). The FS variable in the 20-mg group had significant increases compared to the 10-mg group at 12 months after treatment (P<0.05). The results in Table 2 show the benefit of using rosuvastatin in the treatment of cardiac remodeling and cardiac function.
COMPARISON OF THE OCCURRENCE OF ATRIAL FIBRILLATION:
Two groups of patients were treated with different dosages of rosuvastatin for 12 months. A significant difference in the occurrence rate of atrial fibrillation was observed between the 2 groups. There were 21 cases of paroxysmal atrial fibrillation, 4 cases of persistent atrial fibrillation, and 3 cases of permanent atrial fibrillation in the 10-mg group, for a total of 28 cases (16.0%). There were 8 cases of paroxysmal atrial fibrillation, 2 cases of persistent atrial fibrillation, and 2 cases of permanent atrial fibrillation in 20-mg group, for a total of 12 cases (8.1%). The occurrence rate of AF in the 2 groups was compared by χ2 test, and the difference was significant (P=0.032). In addition, Kaplan-Meier survival analysis showed the difference in the occurrence of AF (log-rank test 4.685, P=0.030) at different dosages of rosuvastatin (Table 3, Figure 3A).
COMPARISON OF THE INCIDENCE OF ISCHEMIC EVENTS OF PATIENTS:
Two groups of patients were treated with different dosages of rosuvastatin for 12 months. There were 21 cases (12.0%) of ischemic events in the group treated with 10 mg of rosuvastatin, including 12 cases of recurring coronary heart disease, 7 cases of heart failure, and 2 cases of stroke. There were 8 cases (5.4%) of ischemic events in the 20-mg group, including 6 cases of recurring coronary heart disease, 1 case of heart failure, and 1 case of stroke. There were no sudden cardiac deaths in these 2 groups. The incidence of ischemic events in the 2 groups was compared by χ2 test. The incidence of ischemic events was significantly different between the 2 groups (P=0.039). In addition, Kaplan-Meier survival analysis also showed the difference (log-rank test 4.277, P=0.039) at different dosages of rosuvastatin (Table 3, Figure 3B).
COMPARISON OF THE ADVERSE CLINICAL EVENTS OF PATIENTS:
Adverse clinical events variables included myalgia and creatine kinase (CK), alanine aminotransferase (ALT), and creatinine (Cr). Two groups of patients were treated with different dosages of rosuvastatin for 12 months. There were 5 unsafe events in the 10-mg group and 4 in the 20-mg group. The data were tested by χ2 test. Table 3 shows there was no difference between the 2 groups (P=0.799), suggesting that it was safe to use 20 mg rosuvastatin for 12 months in clinical practice. Kaplan-Meier survival analysis also showed the same result (log-rank test 0.007, P=0.933) (Figure 3C).
MULTIVARIATE COX REGRESSION ANALYSIS OF RISK FACTORS FOR THE OCCURRENCE OF AF AND ISCHEMIC EVENTS:
We used multivariate Cox regression analysis to investigate the impact of baseline characteristics associated with new-onset AF after AMI. These results demonstrated that different dosages of rosuvastatin (HR=0.330, 95%CI 0.156–0.698, P=0.004), age (HR=1.045, 95%CI 1.006–1.085, P=0.023), smoking (HR=2.680, 95%CI 1.383–5.193, P=0.003), and drinking (HR=2.285, 95%CI 1.154–4.525, P=0.018) are independent factors associated with occurrence of AF. Different dosages of rosuvastatin (HR=0.303, 95%CI 0.128–0.720, P=0.007), diabetes mellitus (HR=2.953, 95%CI 1.169–7.462, P=0.022), and smoking (HR=2.661, 95%CI 1.230–5.755, P=0.013) are independent factors associated with occurrence of ischemic events (Table 4).
Discussion
Statins are the basic drugs used for the treatment of ACS. The optimal dose and safety of statin on patients with ACS have been a hot topic in basic research and clinic practice, especially for rosuvastatin. The results of our study indicated that the administration of rosuvastatin can not only reduce total serum cholesterol and LDL, but also can reduce the incidence rate of new-onset AF and ischemic events in AMI patients. There were no significant differences in the safety of using 2 different doses of rosuvastatin 12 months after admittance. It was found that atrial ischemia, increased left atrial pressure, left atrial enlargement, and left ventricular dysfunction result in ACS and can lead to AF [7]. Previous studies have demonstrated that the mechanisms of rosuvastatin include endothelial function improvement, oxidative stress reduction, and regulation of inflammation and antiarrhythmic effects [24]. The function of the cardiac autonomic nervous system in AF has been of interest in recent years. The imbalanced and inhomogeneous distribution of the sympathetic and pneumogastric nerves is associated with the occurrence and maintenance of AF. A recent study observed the effects of rosuvastatin on effective atrial refractory pacing, the induction of atrial fibrillation, and changes in atrial autonomic nerves in an AF model induced by rapid pacing in rabbits. The results showed that persistent rapid atrial pacing can cause heterogeneous autonomic neural remodeling in different parts of the atrium and can lead to AF, which can be reversed by rosuvastatin [25]. Huang et al. found that in patients receiving statin in clinical practice, the CHADS2 score can help identify AMI patients who will benefit from the use of statin to prevent AF [26]. The risk of new-onset AF was decreased by statin use within 1 month after discharge in patients with myocardial infarction or coronary revascularization [27]. According to Pierri et al., 7-day preoperative treatment with statin decreased the incidence of AF after surgery [28]. Recent evidence showed that statins can decrease the risk of stroke proportional to the degree of cholesterol reduction by lowering TC and LDL-C [29,30].
The main pathological feature of atherosclerosis is the deposition of lipid in some parts of the artery, accompanied by smooth muscle cells proliferation and fibrous matrix components. Factors that contribute to atherosclerotic plaque instability include hemodynamic changes, stress, and inflammation [31]. Inflammatory reactions play an important role in unstable plaque and plaque rupture. Statins can not only reduce TC and LDL-C, but can also strengthen collagen formation, thereby increasing plaque stability. Statins contribute to plaque regression by reducing lipid levels [32]. Multivariate Cox analysis in the present study showed that age, smoking, and drinking are independent risk factors associated with the occurrence of AF after adjusting for other factors. Increased plaque instability may be the cause of the incidence of AF, together with smoking, drinking, and older age. Long-term treatment with statins is related to decreased occurrence of plaque rupture detected by OCT in patients presenting with ACS, with a potentially more marked effect in patients with NSTE-ACS [33]. Statins can enhance the production of endothelial-derived NO synthase, increase coronary blood flow, and reduce ischemia reperfusion injury. Our data showed that it can improve heart function by reducing the occurrence of cardiac ischemic events and demonstrated a downward trend of the incidence of AF. The activation of Akt/Nrf2/HO-1 signaling is involved in the suppressive effect of statin on atrial tachypacing-induced cellular remodeling. These results show the protective effect of statin on AF [15]. A meta-analysis of 5 trials with 524 patients was conducted to study the effect of statin agents on the recurrence of AF after electrical cardioversion. Rosuvastatin significantly decreased the frequency of AF recurrence after successful electrical cardioversion [34]. Patel et al. also performed a meta-analysis of 5 randomized and observational studies that reported statins significantly decreased the occurrence rate of AF [35].
The SECURE-PCI trial assessed the impact of using 80 mg atorvastatin on Major Adverse Cardiovascular Events (MACE) before and 24 h after the planned percutaneous coronary intervention (PCI). Results showed that there is a significant 28% relative risk reduction in MACE at 30 days in ACS patients [36]. The PULSAR study demonstrated that 10 mg rosuvastatin treatment was more effective than atorvastatin 20 mg treatment in LDL-C reduction in patients with hypercholesterolemia and there was no significant difference in the occurrence rate of adverse clinical events [37]. It was also reported that equivalent reduction of LDL-C and non-HDL-C required higher dose of atorvastatin and simvastatin than rosuvastatin [38]. High-dose statin pretreatment has an important effect on improving the TIMI flow in patients undergoing PCI, and high-dose statin preloading also reduces the incidence of MACE [39]. High-dose rosuvastatin increases macrophage ATP-binding cassette A1 protein transporter in human atherosclerotic plaque [40]. The results of this study showed that 20 mg per day rosuvastatin treatment can reduce the occurrence rate of ischemic events significantly compared to before treatment. The factors increasing the chance of ischemic events are as follows: male, diabetic, hypertensive, heart failure or occult heart failure, and noncompliance with statins. High-dose statin therapy should be given as soon as possible after admission for AMI patients. However, patients with long-term use of high-dose statins may develop rhabdomyolysis, liver and/or kidney damage and other adverse reactions. During the 12-month follow-up in this study, the clinical and blood biochemical variables of the patients were observed. The results showed that the adverse clinical events were not significantly different between the 10-mg per day rosuvastatin group and the 20-mg per day group at 12 months after admittance. These results suggest that long-term use of 20 mg per day rosuvastatin is safe. Approximately 10% of the dose of rosuvastatin is metabolized by hepatic cytochrome P4502C9. Approximately 90% of it is excreted in the stools (including absorption and active substances not absorbed), while the rest is excreted through the urine. The plasma clearance half-life of rosuvastatin is about 19 h. The half-life of a clearance did not increase with the increase of dose. However, the use of high doses of statins in elderly patients with multiple chronic diseases and liver and/or kidney dysfunction may increase the risk of adverse effects. Our 20-mg per day rosuvastatin group had fewer adverse clinical events, but it is possible the 12-month observation period was not long enough to detect significant differences between the 2 groups. Additionally, longer-term and larger-size clinical observation should be performed to determine whether any additional benefits could be demonstrated. The newly listed lipid-lowering drug PCSK9 inhibitor is a new development in the field of lipid management [41], but it is much more expensive than generic statins, and its cost may impede its clinical application and reduce long-term adherence. Therefore, it is more practical to use less expensive statins for clinical treatment of hypercholesterolemia [42–44].
Conclusions
Rosuvastatin can significantly reduce serum lipid levels, improve heart function, and reduce the incidence of AF and other cardiovascular events in AMI patients with new-onset AF. The incidence of adverse clinical events was similar in the 2 groups. Smoking and drinking are risk factors for the occurrence of AF and ischemic events. The clinical use of 20 mg rosuvastatin is medically safe within 12 months after hospitalization.
Figures
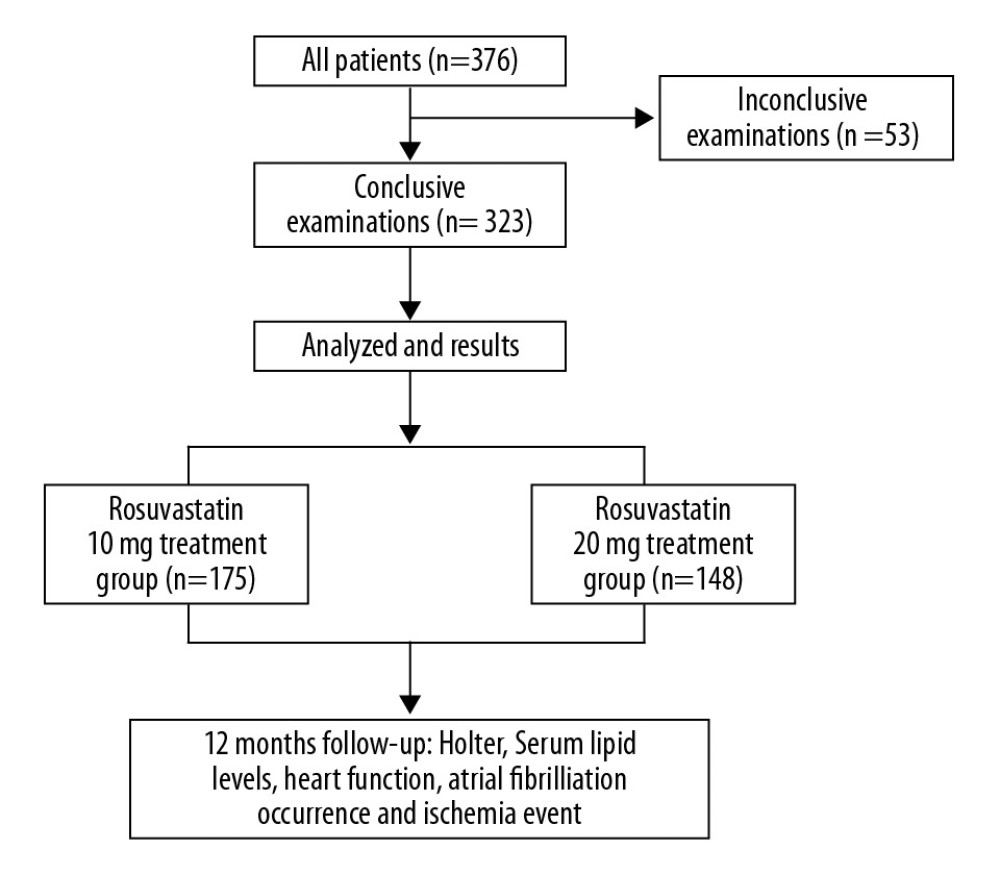 Figure 1. The flow diagram of the main procedures.
Figure 1. The flow diagram of the main procedures. 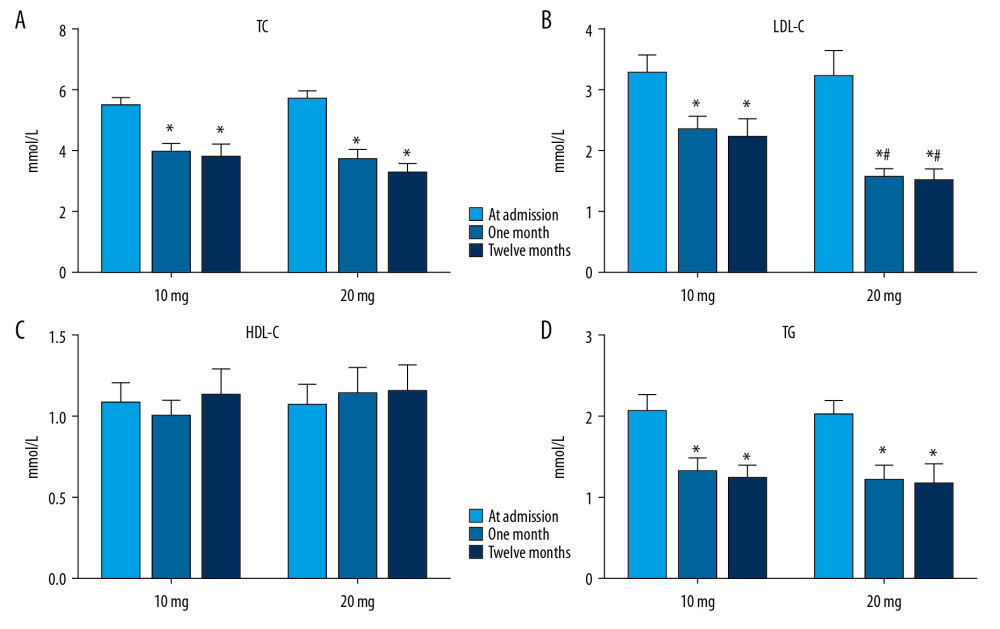 Figure 2. Serum lipid levels at admission and at 1 and 12 months after treatment with different dosages of rosuvastatin. Data were compared using ANOVA followed by Tukey’s test. (A) TC; (B) LDL-C; (C) HDL-C; and (D) TG. Mean±SEM, * P<0.05 vs. at admission, # P<0.05 vs. 10-mg group.
Figure 2. Serum lipid levels at admission and at 1 and 12 months after treatment with different dosages of rosuvastatin. Data were compared using ANOVA followed by Tukey’s test. (A) TC; (B) LDL-C; (C) HDL-C; and (D) TG. Mean±SEM, * P<0.05 vs. at admission, # P<0.05 vs. 10-mg group. 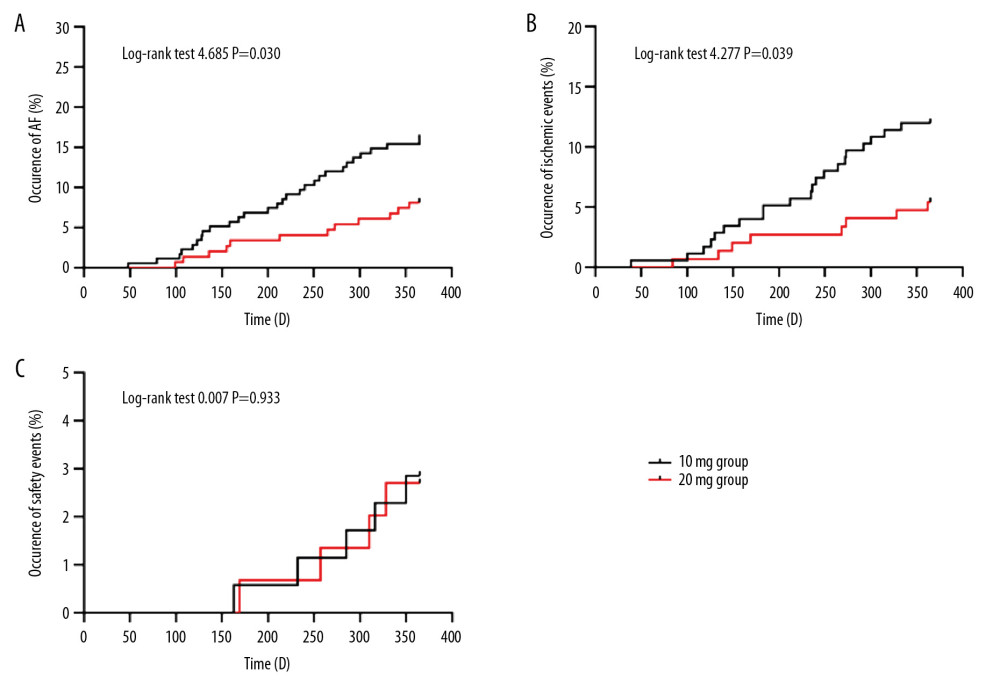 Figure 3. Comparison of occurrence of atrial fibrillation (A), ischemic events (B), and adverse clinical events (C) after 12 months of treatment with different dosages of rosuvastatin. Data were compared by log-rank test for the Kaplan-Meier curve.
Figure 3. Comparison of occurrence of atrial fibrillation (A), ischemic events (B), and adverse clinical events (C) after 12 months of treatment with different dosages of rosuvastatin. Data were compared by log-rank test for the Kaplan-Meier curve. Tables
Table 1. Baseline characteristics of the two groups.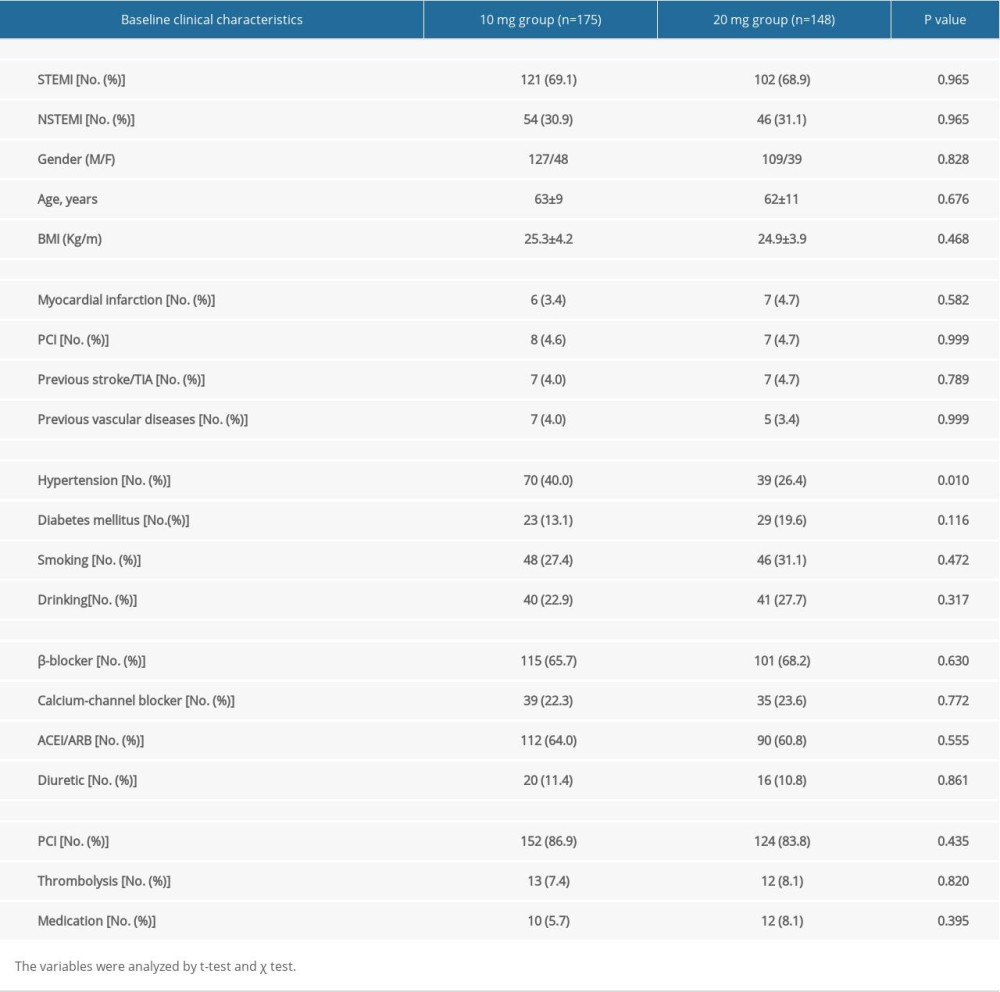 Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin.
Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin.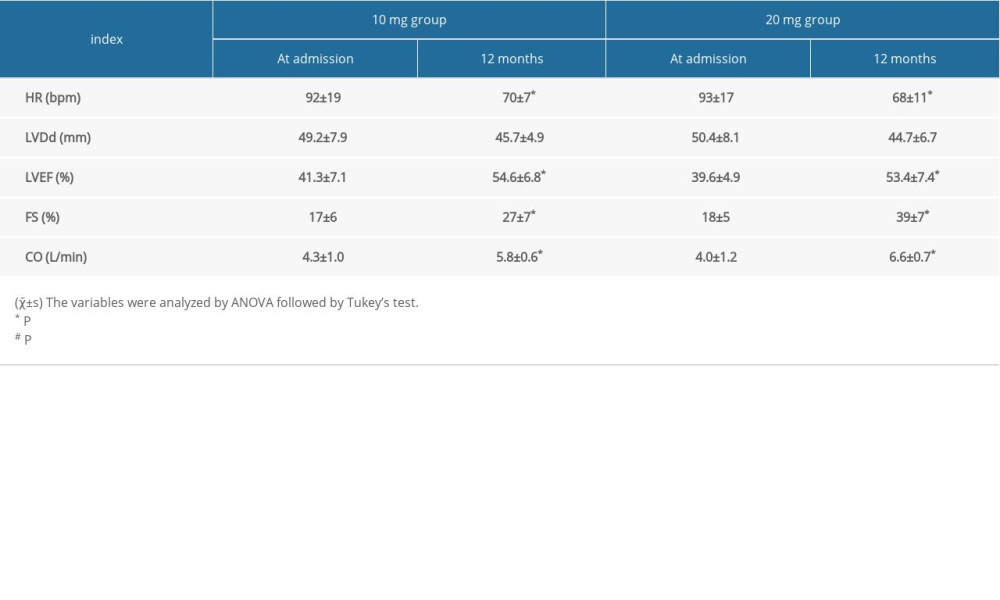 Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin.
Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin.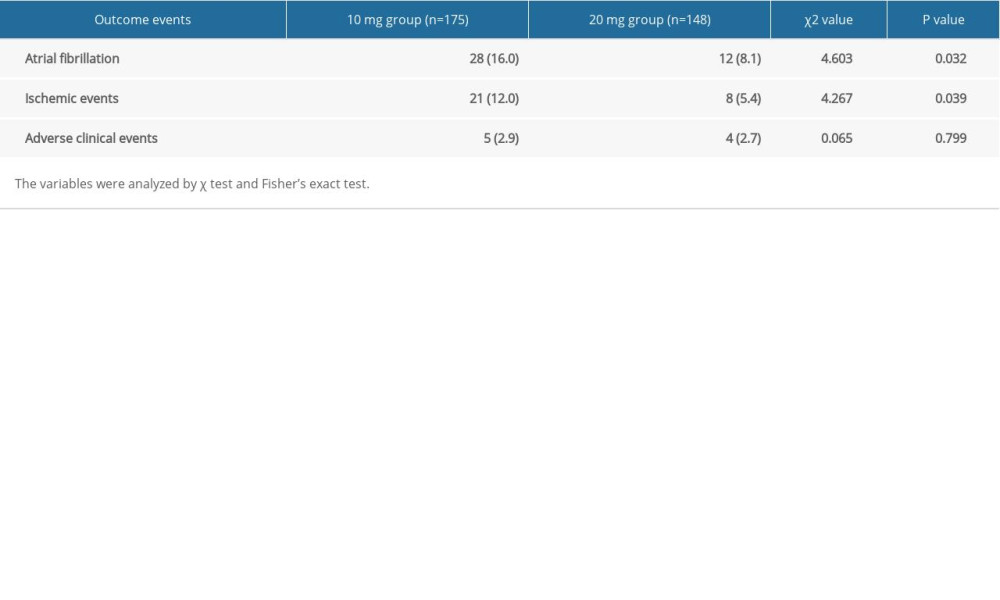 Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events.
Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events.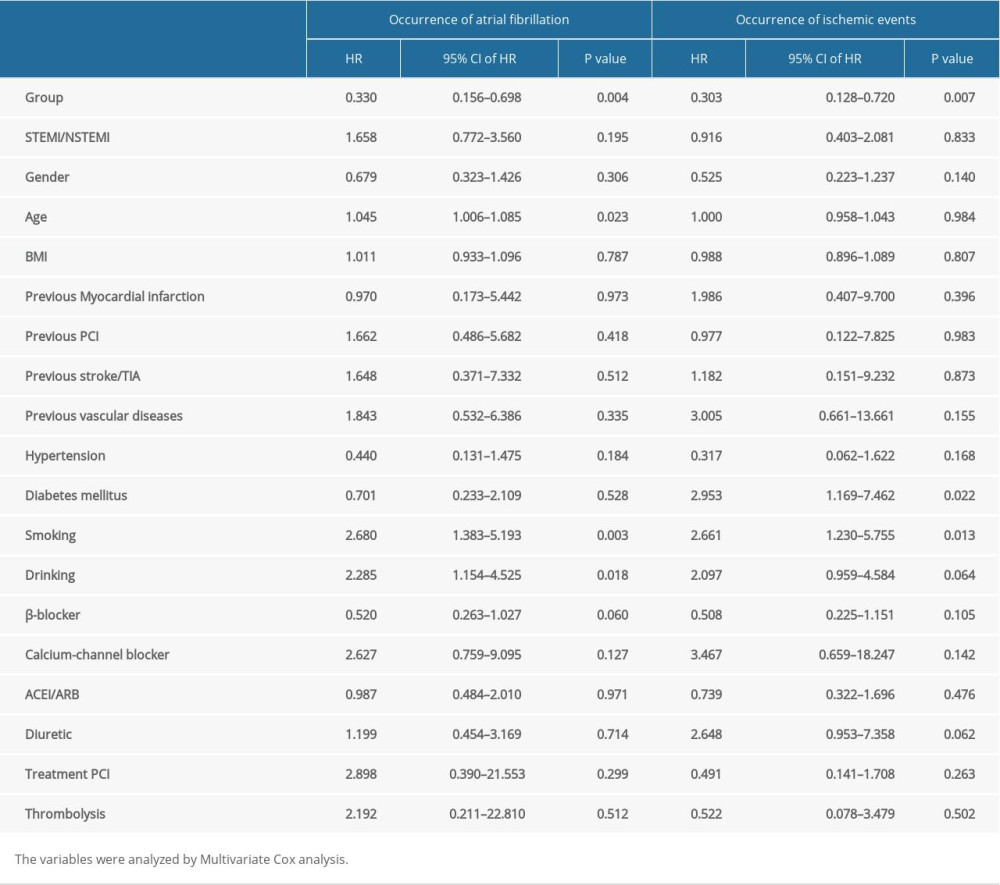
References
1. Kundu A, O’Day K, Shaikh AY, Relation of atrial fibrillation in acute myocardial infarction to in-hospital complications and early hospital readmission: Am J Cardiol, 2016; 117; 1213-18
2. Bang CN, Gislason GH, Greve AM, Statins reduce new-onset atrial fibrillation in a first-time myocardial infarction population: A nationwide propensity score-matched study: Eur J Prev Cardiol, 2014; 21; 330-38
3. Congo KH, Belo A, Carvalho J, New-onset atrial fibrillation in St-segment elevation myocardial infarction: Predictors and impact on therapy and mortality: Arq Bras Cardiol, 2019; 113; 948-57
4. Guenancia C, Toucas C, Fauchier L, High rate of recurrence at long-term follow-up after new-onset atrial fibrillation during acute myocardial infarction: Europace, 2018; 20; e179-88
5. Consuegra-Sanchez L, Melgarejo-Moreno A, Galcera-Tomas J, Short- and long-term prognosis of previous and new-onset atrial fibrillation in ST-segment elevation acute myocardial infarction: Rev Esp Cardiol (Engl Ed), 2015; 68; 31-38
6. Schmitt J, Duray G, Gersh BJ, Hohnloser SH, Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications: Eur Heart J, 2009; 30; 1038-45
7. Stamboul K, Fauchier L, Gudjoncik A, New insights into symptomatic or silent atrial fibrillation complicating acute myocardial infarction: Arch Cardiovasc Dis, 2015; 108; 598-605
8. Grundy SM, Stone NJ, Bailey AL, 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: J Am Coll Cardiol, 2019; 73; e285-350
9. Mach F, Baigent C, Catapano AL, 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: Eur Heart J, 2020; 41; 111-88
10. Arnett DK, Blumenthal RS, Albert MA, 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: Circulation, 2019; 140; e596-646
11. Goff DC, Cushman WC, Blood-pressure and cholesterol lowering in the HOPE-3 trial: N Engl J Med, 2016; 375; 1194
12. Sexton TR, Wallace EL, Macaulay TE, The effect of rosuvastatin on thromboinflammation in the setting of acute coronary syndrome: J Thromb Thrombolysis, 2015; 39; 186-95
13. Zhao Y, Peng R, Zhao W, Zhibitai and low-dose atorvastatin reduce blood lipids and inflammation in patients with coronary artery disease: Medicine, 2017; 96; e6104
14. Wu S, Yang R, Wang G, Anti-asthmatic effect of pitavastatin through aerosol inhalation is associated with CD4+ CD25+ Foxp3+ T cells in an asthma mouse model: Sci Rep, 2017; 7; 6084
15. Yeh YH, Kuo CT, Chang GJ, Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway: J Mol Cell Cardiol, 2015; 82; 84-92
16. Yang F, Wang J, Li F, Cui L, Atorvastatin combined nitroglycerin therapy confer additive effects on rabbits with dyslipidemia: Exp Clin Endocrinol Diabetes, 2016; 124; 367-71
17. Yuan X, Du J, Liu Q, Zhang L, Defining the role of perioperative statin treatment in patients after cardiac surgery: A meta-analysis and systematic review of 20 randomized controlled trials: Int J Cardiol, 2017; 228; 958-66
18. Liang M, Yang S, Fu N, Efficacy of short-term moderate or high-dose rosuvastatin in preventing contrast-induced nephropathy: A meta-analysis of 15 randomized controlled trials: Medicine, 2017; 96; e7384
19. Ye Z, Lu H, Su Q, Effect of high-dose rosuvastatin loading before percutaneous coronary intervention in Chinese patients with acute coronary syndrome: A systematic review and meta-analysis: PLoS One, 2017; 12; e0171682
20. Jneid H, Anderson JL, Wright RS, 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines: Circulation, 2012; 126; 875-910
21. O’Gara PT, Kushner FG, Ascheim DD, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Circulation, 2013; 127; e362-425
22. Zhao P, Zhang J, Yin XG, The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy: Life Sci, 2013; 92; 633-38
23. Zhang J, Zhang X, Cui Y, Different postconditioning cycles affect prognosis of aged patients undergoing primary percutaneous coronary intervention: Cardiol J, 2018; 25; 666-73
24. Abaci O, Kocas C, Oktay V, Comparison of rosuvastatin versus atorvastatin for preventing postoperative atrial fibrillation: Heart Surg Forum, 2013; 16; E158-61
25. Zhang B, Wang X, Li Z, Atrial heterogeneous autonomic neural remodeling in rabbits with experimental atrial fibrillation and the effect of intervention by rosuvastatin: Pacing Clin Electrophysiol, 2016; 39; 598-606
26. Huang SS, Chan WL, Leu HB, Association between CHADS2 score and the preventive effect of statin therapy on new-onset atrial fibrillation in patients with acute myocardial infarction: PLoS One, 2013; 8; e74709
27. Kulik A, Singh JP, Levin R, Association between statin use and the incidence of atrial fibrillation following hospitalization for coronary artery disease: Am J Cardiol, 2010; 105; 1655-60
28. Pierri MD, Crescenzi G, Zingaro C, Prevention of atrial fibrillation and inflammatory response after on-pump coronary artery bypass using different statin dosages: A randomized, controlled trial: Gen Thorac Cardiovasc Surg, 2016; 64; 395-402
29. Akioyamen LE, Tu JV, Genest J, Risk of ischemic stroke and peripheral arterial disease in heterozygous familial hypercholesterolemia: a meta-analysis: Angiology, 2019; 70; 726-36
30. Salvatore T, Morganti R, Marchioli R, De Caterina R, Cholesterol lowering and stroke: No longer room for pleiotropic effects of statins – confirmation from PCSK9 inhibitor studies: Am J Med, 2020; 133; 95-99.e6
31. Parma L, Baganha F, Quax PHA, de Vries MR, Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis: Eur J Pharmacol, 2017; 816; 107-15
32. Almeida SO, Budoff M, Effect of statins on atherosclerotic plaque: Trends Cardiovasc Med, 2019; 29; 451-55
33. Gili S, Iannaccone M, Colombo F, Effects of statins on plaque rupture assessed by optical coherence tomography in patients presenting with acute coronary syndromes: Insights from the optical coherence tomography (OCT)-FORMIDABLE registry: Eur Heart J Cardiovasc Imaging, 2018; 19; 524-31
34. Yan P, Dong P, Li Z, Cheng J, Statin therapy decreased the recurrence frequency of atrial fibrillation after electrical cardioversion: A meta-analysis: Med Sci Monit, 2014; 20; 2753-58
35. Patel AA, White CM, Shah SA, The relationship between statin use and atrial fibrillation: Curr Med Res Opin, 2007; 23; 1177-85
36. Berwanger O, Santucci EV, de Barros E, Silva PGM, Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: The SECURE-PCI randomized clinical trial: JAMA, 2018; 319; 1331-40
37. Clearfield MB, Amerena J, Bassand JP, Comparison of the efficacy and safety of Rosuvastatin 10 mg and Atorvastatin 20 mg in high-risk patients with hypercholesterolemia – Prospective Study to Evaluate the Use of Low Doses of the Statins Atorvastatin and Rosuvastatin (PULSAR): Trials, 2006; 7; 35
38. Karlson BW, Palmer MK, Nicholls SJ, Doses of Rosuvastatin, Atorvastatin and Simvastatin that induce equal reductions in LDL-C and non-HDL-C: Results from the VOYAGER meta-analysis: Eur J Prev Cardiol, 2016; 23; 744-47
39. Xiao Y, He S, Zhang Z, Effect of high-dose statin pretreatment for myocardial perfusion in patients receiving percutaneous coronary intervention (PCI): A meta-analysis of 15 randomized studies: Med Sci Monit, 2018; 24; 9166-76
40. Santovito D, Marcantonio P, Mastroiacovo D, High dose rosuvastatin increases ABCA1 transporter in human atherosclerotic plaques in a cholesterol-independent fashion: Int J Cardiol, 2020; 299; 249-53
41. Sabatine MS, Giugliano RP, Keech AC, Evolocumab and clinical outcomes in patients with cardiovascular disease: N Engl J Med, 2017; 376; 1713-22
42. Kasichayanula S, Grover A, Emery MG, Clinical pharmacokinetics and pharmacodynamics of evolocumab, a PCSK9 inhibitor: Clin Pharmacokinet, 2018; 57; 769-79
43. Kazi DS, Moran AE, Coxson PG, Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease: JAMA, 2016; 316; 743-53
44. Hlatky MA, Kazi DS, PCSK9 inhibitors: economics and policy: J Am Coll Cardiol, 2017; 70; 2677-87
Figures
 Figure 1. The flow diagram of the main procedures.
Figure 1. The flow diagram of the main procedures. Figure 2. Serum lipid levels at admission and at 1 and 12 months after treatment with different dosages of rosuvastatin. Data were compared using ANOVA followed by Tukey’s test. (A) TC; (B) LDL-C; (C) HDL-C; and (D) TG. Mean±SEM, * P<0.05 vs. at admission, # P<0.05 vs. 10-mg group.
Figure 2. Serum lipid levels at admission and at 1 and 12 months after treatment with different dosages of rosuvastatin. Data were compared using ANOVA followed by Tukey’s test. (A) TC; (B) LDL-C; (C) HDL-C; and (D) TG. Mean±SEM, * P<0.05 vs. at admission, # P<0.05 vs. 10-mg group. Figure 3. Comparison of occurrence of atrial fibrillation (A), ischemic events (B), and adverse clinical events (C) after 12 months of treatment with different dosages of rosuvastatin. Data were compared by log-rank test for the Kaplan-Meier curve.
Figure 3. Comparison of occurrence of atrial fibrillation (A), ischemic events (B), and adverse clinical events (C) after 12 months of treatment with different dosages of rosuvastatin. Data were compared by log-rank test for the Kaplan-Meier curve. Tables
 Table 1. Baseline characteristics of the two groups.
Table 1. Baseline characteristics of the two groups. Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin.
Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin. Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin.
Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin. Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events.
Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events. Table 1. Baseline characteristics of the two groups.
Table 1. Baseline characteristics of the two groups. Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin.
Table 2. Comparison of heart function at admission and after 12 months treatment with different dosages of rosuvastatin. Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin.
Table 3. Comparison of outcome events after 12 months treatment with different dosages of rosuvastatin. Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events.
Table 4. Multivariate COX regression of independent factors for the occurrence of atrial fibrillation and ischemic events. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








