27 October 2020: Clinical Research
Stroke Incidence in Survivors of Carbon Monoxide Poisoning in South Korea: A Population-Based Longitudinal Study
Hyuk-Hoon Kim1ABCE, Sangchun Choi1ADEF*, Yoon Seok Jung1CD, Young-Gi Min1AC, Dukyong Yoon2BC, Sung Eun Lee1DFDOI: 10.12659/MSM.926116
Med Sci Monit 2020; 26:e926116
Abstract
BACKGROUND: Carbon monoxide (CO) poisoning is a suspected risk factor for stroke. However, the association between stroke occurrence and carbon monoxide poisoning remains unclear. This nationwide study in Korea analyzed the incidence of stroke in survivors of CO poisoning.
MATERIAL AND METHODS: In this nationwide, population-based longitudinal study, the database of the Health Insurance Review and Assessment Service was searched to identify patients diagnosed with CO poisoning from 2012 to 2018. Their incidence of ischemic and hemorrhagic strokes, the patterns of stroke incidences, the annual incidence rates in sequential time, the standardized incidence ratio (SIR), and the effects of hyperbaric oxygen therapy (HBOT) were analyzed.
RESULTS: Of the 29 301 patients diagnosed with CO poisoning during the study period, 984 (3.36%) were diagnosed with stroke after CO poisoning, with approximately 50% occurring within 1 year after CO poisoning. The overall SIR for stroke was 19.49 (95% confidence interval [CI], 17.92–21.12) during the first year, decreasing to 5.64 (95% CI, 4.75–6.66) during the second year. Overall stroke hazard ratio (HR) in the patients admitted to the ICU for CO poisoning was 2.28 (95% CI, 1.19–2.27), compared with 2.35 (95% CI, 1.94–2.84) for ischemic stroke and 1.76 (95% CI, 1.11–2.78) for hemorrhagic stroke. Cumulative HRs did not differ between patients who were and were not treated with HBOT for stroke.
CONCLUSIONS: CO poisoning is a high-risk factor for the development of stroke, evidenced by high incidences of stroke after CO poisoning. Practical strategies for preventing stroke after CO poisoning are needed, because stroke after CO poisoning affects adults of almost all ages, significantly increasing their socioeconomic burden.
Keywords: Carbon Monoxide Poisoning, Epidemiology, Risk Factors, Stroke, Korea, Aged, 80 and over, Databases, Factual, Incidence, Longitudinal Studies, Republic of Korea, Survivors, young adult
Background
Stroke may be caused by an obstruction in the flow of oxygen-rich blood to the brain. The signs and symptoms of stroke can vary from a mild headache to death, depending on the degree of severity. Stroke is the leading cause of increases in disability-adjusted life years, with a high socioeconomic burden globally, including in South Korea [1–4]. Attempts have been made to identify risk factors for stroke and to establish potential strategies to prevent the occurrence of stroke in the general population [5–7]. Certain conditions have been reported to temporarily increase the risk of stroke, including trauma (e.g., fractures), preeclampsia, major surgery, and admission to the Intensive Care Unit (ICU) [8–11].
Neurological sequelae can occur after carbon monoxide (CO) poisoning, with several neurological insults thought to be caused by CO toxicity. Cerebral injuries associated with CO have a complex pathophysiology involving hypoxic stress, in which CO plays a significant role; oxidative stress induced by reactive oxygen species; and lipid peroxidation. These conditions can lead to the demyelination of white matter, with a catecholamine crisis followed by the release of dopamine and glutamate in excessive amounts [12,13]. Resulting pathological changes are visible on brain magnetic resonance imaging (MRI) as hippocampal necrosis, the demyelination of cerebral white matter, and vacuolar necrosis of the cerebral cortex and globus pallidus [14].
Although several studies have assessed the long-term effects of CO poisoning on the incidence of ischemic stroke, none to our knowledge has assessed the occurrence of all types of stroke after CO poisoning [15–17]. The present study was designed to analyze nationwide occurrence of stroke in survivors of CO poisoning throughout South Korea. This longitudinal follow-up study was based on national claims data from the Health Insurance Review and Assessment (HIRA) Service in South Korea. The HIRA data, also called National Health Insurance (NHI) data, consist of claims for healthcare services submitted for reimbursement under the NHI system in South Korea [18].
Material and Methods
DATA SOURCE:
The NHI is the public medical insurance system in South Korea, providing health insurance coverage for more than 99% of the nation’s population. The HIRA Service database, based on the NHI system, contains patients’ demographic information (e.g., sex, age, and area of residence) and clinical details (e.g., diagnoses, procedures, and prescriptions). The nationwide cohort in the present study, defined based on the HIRA claims data, consisted of all patients diagnosed with CO poisoning from 2012 to 2018. Data were extracted by a data manager of the HIRA who works in the NHI, with most data analyzed by an emergency physician who completed a certification program in biomedical informatics for physicians, hosted by the Korean Society of Medical informatics. Finally, the analytical results were validated by a professor in the Department of Biomedical Informatics of the university-affiliated hospital, in which this study was conducted. The study protocol was approved by the institutional review board (IRB) of Ajou University Medical Center (IRB no. AJIRB-MED-EXP-19-563), which waived informed consent because all data in this study were obtained retrospectively and de-identified.
STUDY DESIGN AND STUDY PARTICIPANTS:
The study participants consisted of all patients diagnosed with CO poisoning from January 1, 2007, to December 31, 2018. The diagnostic code was T58, based on the Korean Classification of Disease, sixth edition, a modified version of the International Classification of Disease-10 (ICD-10) for the Korean health care system. Strokes were diagnosed using either a single imaging modality (e.g., computed tomography and brain MRI) or both. Diagnostic codes for stroke included subarachnoid hemorrhage (I60), intracerebral hemorrhage (I61), other non-traumatic intracranial hemorrhages (I62), and cerebral infarction (I63). The records of patients who experienced strokes after CO poisoning were identified using the primary or secondary disease code in the claims data generated during the first inpatient or outpatient visit. Procedure codes assigned by the intensivist on admission to the ICU (i.e., AJ100-AJ190, AJ200-AJ290, and AJ300-AJ390, or AJ001) were used to validate the ICU admission [20].
Patients with a history of CO poisoning occurring before January 1, 2012, were excluded to remove any possible bias associated with a previous account of CO poisoning. Patients with a history of stroke before January 1, 2012, were also excluded, as this study focused on newly occurring strokes after CO poisoning. Additionally, patients diagnosed with stroke before CO poisoning were excluded, even those diagnosed between 2012 and 2018.
STATISTICAL ANALYSIS:
Descriptive statistics were used to estimate the frequency of strokes by diagnostic date, based on the first hospital visit. The annual incidence rate was defined as the number of patients newly diagnosed with strokes divided by the sum of all patients in the study population during the entire follow-up period. Additionally, standardized incidence ratios (SIRs) and their corresponding 95% confidence intervals (CIs) were calculated to assess the relative risk of first-time stroke by stroke types and by year chronologically after CO poisoning. These findings were compared with those in the general population of South Korea, in which incidence rate of stroke was 216 per 100 000/year, according to the official stroke registry database [2]. The CIs were calculated according to normal approximations, with the mid-p exact test used for the patients with fewer than 5 observations [19]. The occurrence of stroke after CO poisoning was estimated using the Kaplan-Meier method, with groups admitted and not admitted to the ICU and those who did and did not receive hyperbaric oxygen therapy (HBOT) at least once over 30 minutes during the initial admission period compared by log-rank tests. A 2-sided P value ≤0.05 indicated statistical significance, and all statistical analyses were performed using R, version 3.0.2 (R Development Core Team, Vienna, Austria).
Results
PATTERNS OF STROKE INCIDENCE AFTER CO POISONING:
Out of the 29 301 patients diagnosed with CO poisoning, the baseline characteristics of enrolled patients are shown on Table 1, 984 (3.36%) were diagnosed with stroke after the median years of 3.07 (range, 0.00–6.99) after the CO poisoning (Figure 1). The most common type of stroke after CO poisoning was ischemic stroke (813, 82.62%), followed by intracranial hemorrhagic (ICH) stroke (114, 11.59%) and subarachnoid hemorrhagic (SAH) stroke (43, 4.37%). About 50% of each type of stroke occurred within the first year after the CO poisoning. The incidence of ischemic stroke differed significantly in patients with (11.27% [56/497]) and without (2.37% [683/28 804]) a history of heart failure (P<0.001); and between patients with (11.62% [33/284]) and without (2.43% [706/29 017]) a history of relevant atrial dysrhythmia (P<0.001).
DISTRIBUTION OF CO POISONING PATIENTS AND STROKE INCIDENCE RATE:
Figure 2A shows the numbers of patients diagnosed with CO poisoning by age and sex. Of the patients diagnosed with CO poisoning, 56.47% were male and 43.53% were female. However, the highest rates of CO poisoning were observed in females aged <20 years and ≥70 years. The total number of patients with CO poisoning was highest in patients aged 30–39 years. The annual stroke incidence rate tended to increase with age and peaked in patients aged 70–79 years. The crude stroke SIR for patients after CO poisoning was 14.48 (95% CI, 13.58–15.41), including 18.92 (95% CI, 14.73–16.70) for patients aged ≤54 years, 22.98 (95% CI, 20.85–25.28) for patients aged 55–74 years, and 34.59 (95% CI, 30.19–39.46) for patients aged ≥75 years (Figure 2B).
SEQUENTIAL RATES OF ANNUAL STROKE INCIDENCES AFTER CO POISONING BY STROKE TYPE:
In the first year after CO poisoning, the overall stroke SIR was 19.47 (95% CI, 17.92–21.12), decreasing markedly during the second year to 5.64 (95% CI, 4.75–6.66). The incidence risk for all types of strokes decreased over time. During the fourth year after CO poisoning, however, secondary peak SIR patterns were observed for ischemic stroke and ICH stroke, but not for SAH stroke. Although the risk of hemorrhagic stroke decreased to that of the general population by the sixth year (SIR, 2.07; 95% CI, 0.56–5.29), the risk of ischemic stroke remained higher than that of the general population, even in the seventh year after CO poisoning (SIR, 3.25; 95% CI, 1.19–7.07) (Table 2).
STROKE INCIDENCES AFTER CO POISONING ACCORDING TO PREMORBID STATUS:
Overall SIR for stroke in patients poisoned with CO was 1.84 (95% CI, 1.55–2.17). Several premorbid conditions, including diabetes mellitus, hypertension, heart failure, atrial fibrillation, ischemic heart disease, and depressive disorders, all of which are recognized as risk factors for stroke incidence, were associated with higher SIR. Among these premorbid statuses, atrial fibrillation (SIR 7.12) and heart failure (SIR 6.94) were associated with the highest risks of stroke (Table 3).
RISK OF STROKE FOR PATIENTS ADMITTED TO THE ICU AND FOR THOSE WHO RECEIVED HBOT:
ICU admission due to CO poisoning was significantly associated with the development of stroke after CO poisoning. The cumulative hazard ratio (HR) for overall stroke in patients admitted to the ICU due to CO poisoning was 2.28 (95% CI, 1.19–2.27), being 2.35 (95% CI 1.94–2.84) for ischemic stroke and 1.76 (95% CI 1.11–2.78) for hemorrhagic stroke (Figure 3). In contrast, the cumulative HRs for incidences of all types of strokes did not differ significantly in patients who did and did not receive HBOT (Figure 4).
Discussion
The findings of this study indicate that the incidence of stroke after CO poisoning was highest during the first and second years, regardless of the type of stroke. This incidence tended to decrease over time until it was equal to that of the general population after about 7 years. Because CO poisoning tends to occur more frequently in younger people, whereas the incidence rate of stroke is higher in older people, the incidence of stroke after CO was higher in both younger and older adults than in middle-aged persons. The risk of stroke was also higher in patients who were than were not admitted to the ICU due to CO poisoning, but did not differ significantly between patients who did and did not receive HBOT. These findings suggest that active prevention is necessary to reduce the incidence rate of stroke after acute CO poisoning.
This study contrasts with previous studies for several reasons. First, this retrospective study established a disease-free period of 5 years to eliminate any bias from diseases diagnosed previously. The patients were followed up for almost 7 years after CO poisoning, during which the annual incidence rates for all types of strokes were analyzed. Second, we compared risks of stroke after CO poisoning in patients grouped by age, sex, ICU admission, and HBOT, yielding results comparable to those of a recent epidemiologic study based on the nationwide representative stroke registry in South Korea [2]. Third, variations in follow-up times for scheduling may have minimized the temporal bias stemming from stroke-related issues, such as the insurance curtailment for brain magnetic resonance imaging and the increased social concern about stroke.
Of the patients who experienced CO poisoning, 984 (3.39%) experienced strokes. The stroke incidence rate was high in younger patients, being highest in patients aged 30–39 years, followed by those aged 40–49 years. Although the risk of stroke increased with age, more patients aged <54 years than aged >75 years developed stroke after CO poisoning. Because the incidence of CO poisoning is higher in younger patients, stroke following by CO poisoning contributes substantially to an increase in the national socioeconomic burden. Therefore, effective strategies for the active prevention of CO poisoning, as well as for subsequent stroke, are required.
Patterns of stroke occurrence after CO poisoning differed across the types of stroke. The incidence of stroke, regardless of type, was highest during the first and the second years after CO poisoning, subsequently decreasing over time. Ischemic stroke was the most frequent type of stroke, followed by ICH and SAH types. The risk of developing ischemic stroke remained higher than in the general population for up to 7 years after CO poisoning, whereas the risks of ICH and SAH strokes were higher for only 5 and 2 years, respectively. These differences in patterns of stroke incidence and risk factors are attributable to differences in the pathophysiology of these different types of stroke following CO toxicity. The high rate of ischemic stroke soon after CO poisoning may be associated with a pro-thrombotic tendency resulting from CO-induced pro-coagulant features and hypo-fibrinolysis [21]. In addition, cerebrovascular endothelial dysfunction or endothelial cell death induced by oxidative stress could also contribute to the development of ischemic stroke after CO poisoning [22,23]. CO toxicity may also increase the risk of ischemic stroke in patients with existing cardiac risk factors, such as heart failure and relevant atrial dysrhythmias, including atrial fibrillation, atrial flutter, aggravated atrial dysrhythmias and heart failure [24,25].
Vessel injuries in the central nervous system may result from a disruption of microvascular endothelial integrity caused by excessive increases in superoxide, nitric oxide, and peroxynitrite. These increases may be caused by ischemia/reperfusion injury in patients with CO poisoning, resulting in the development of ICH stroke [26]. However, because stroke occurs soon after CO poisoning, followed by a rapid decrease of risk during the second year, the development of SAH may result from events during the initial stage of the CO poisoning process, such as an epinephrine-induced catecholamine surge of CO-induced dysregulation of cerebral blood control [24,27]. These phenomena may increase intracranial pressure, affecting existing cerebral aneurysms, or may directly induce aneurysmal changes in cerebral arteries. If these resultant aneurysms do not remain stable, they can eventually lead to SAH stroke after CO poisoning. The secondary peaks in the incidence patterns of ischemic stroke and ICH stroke are thought to be consequences of the long-term effects of endothelial dysfunction and disruption, causing subtle changes in the cerebral micro-vessels if they do not undergo spontaneous repair.
Because of the high risk of stroke after CO poisoning, particularly in young patients, and the burden associated with stroke-related diseases, strategies are needed to prevent stroke after CO poisoning. The current treatment guidelines for acute CO poisoning include HBOT to reduce the severity of acute complications as well as the delayed neurological sequelae in patients with severe CO poisoning. However, no study to date has evaluated the effects of HBOT on stroke development after CO poisoning. Our study found that stroke incidence after CO poisoning did not differ between those who did and did not receive HBOT. HBOT, however, may be useful for reducing stroke occurrence in patients admitted to the ICU for CO poisoning. Our finding, that stroke after CO poisoning was more frequent in patients with pre-existing heart failure, atrial fibrillation, or atrial flutter, suggests that antiplatelet therapy may prevent stroke after CO poisoning. Other methods of risk factor management, including psychiatric intervention, should be considered.
The present study had several limitations. First, CO poisoning and stroke were based on claims data from the HIRA, which contains only disease codes and demographic information. Thus, clinical data were unavailable, such as the source and severity of CO poisoning, the intentionality of poisoning, history of exposure to other substances, and the degree of stroke. Future studies should integrate HIRA claims data with clinical datasets. Second, although the risks for stroke after CO poisoning in this study were compared with data from the nationwide stroke registry in South Korea, any gap in time between the 2 studies may have limited the accuracy of the comparison. This limitation may have had a minimal effect on the results of this study because there have been few variations in stroke incidences over time in South Korea. This limitation may be overcome, however, by well-designed cohort studies evaluating the risk of stroke after CO poisoning. Third, the disease codes for CO poisoning and stroke were based on claims data weighted toward a clinical environment, not a research setting. Diagnoses based on ICD-10 codes are not as accurate as those determined by structured clinical interviews. Validation of the outcome of this study, i.e., the diagnosis of stroke, was questionable because of the lack of clinical data, such as the results of neurologic examinations and imaging modalities. Thus, delayed neurological syndrome-like conditions might have been misdiagnosed as stroke, resulting in the overcounting of outcomes. However, recent national claims-based studies suggested that analyzing the data extracted from nationwide claim datasets produce relatively accurate results. Thus, using HIRA claims data likely did not introduce a significant bias. More definitive studies should be conducted in the future. Fourth, because of the lack of information about other non-traumatic intracranial hemorrhages (I62), results such as the number and SIR of other non-traumatic intracranial hemorrhages were missing.
Conclusions
CO poisoning is a high-risk factor for the development of stroke, as evidenced by high rates of stroke after CO poisoning. Practical strategies for preventing stroke after CO poisoning are needed. Most strokes occur during the first 2 years after CO poisoning and affect patients of all ages, enhancing socioeconomic burden.
Figures
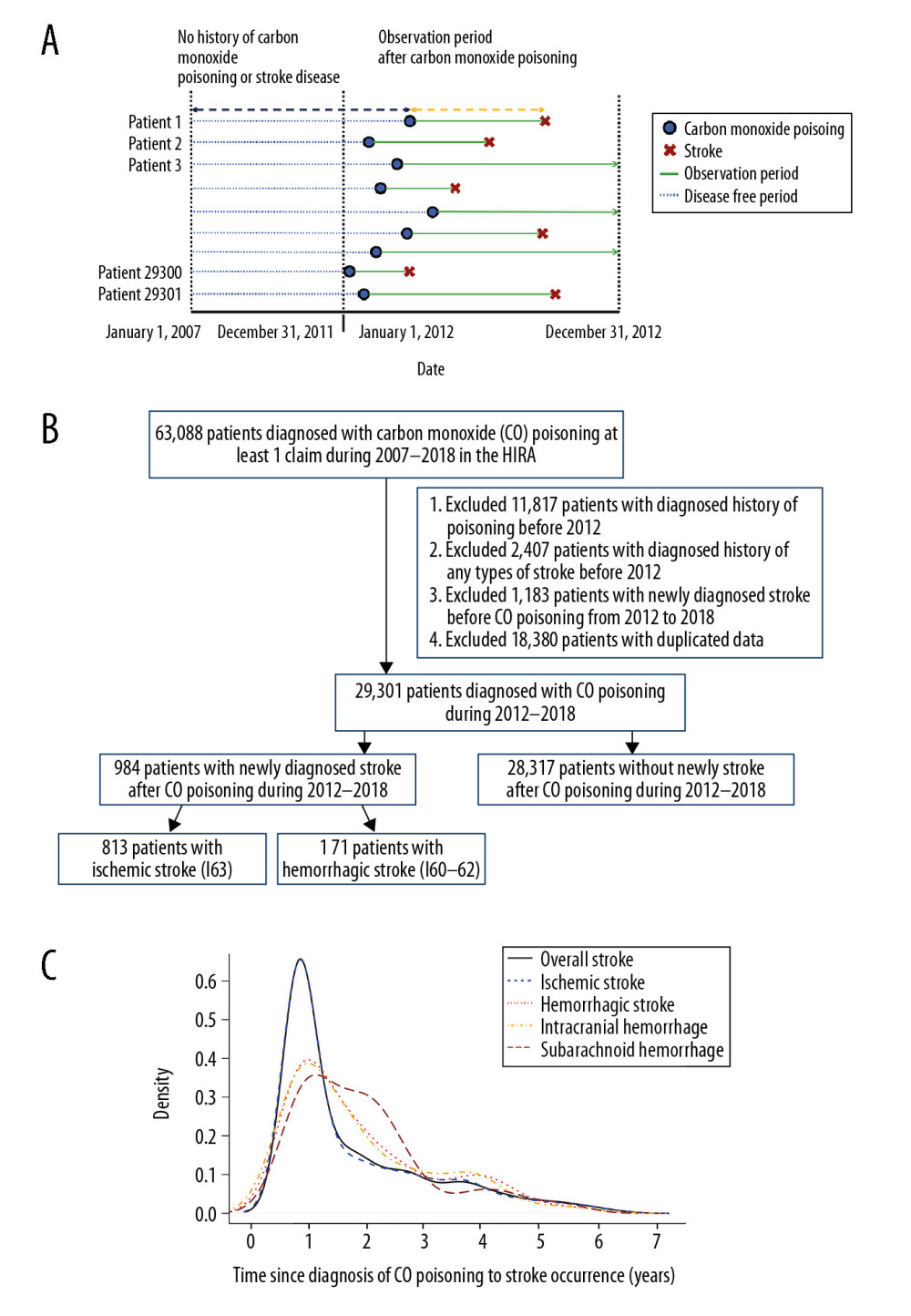 Figure 1. (A) Research design of this retrospective cohort study. Patients diagnosed with CO poisoning from January 1, 2012, to December 31, 2018, were followed up to determine the occurrence of stroke. (B) Patient selection process. Of the 29 301 patients diagnosed with CO poisoning from 2012 to 2018, 984 (3.36%) were subsequently diagnosed with stroke. (C) Frequency of stroke after CO poisoning in survivors. Most strokes occurred during the first 2 years after CO poisoning.
Figure 1. (A) Research design of this retrospective cohort study. Patients diagnosed with CO poisoning from January 1, 2012, to December 31, 2018, were followed up to determine the occurrence of stroke. (B) Patient selection process. Of the 29 301 patients diagnosed with CO poisoning from 2012 to 2018, 984 (3.36%) were subsequently diagnosed with stroke. (C) Frequency of stroke after CO poisoning in survivors. Most strokes occurred during the first 2 years after CO poisoning. 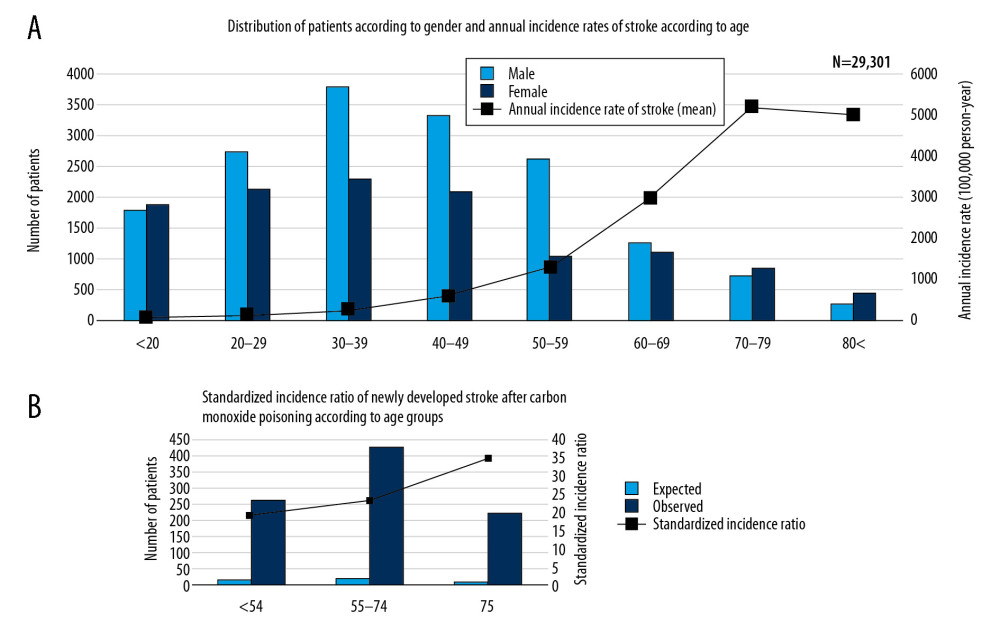 Figure 2. Overall distribution of patients with carbon monoxide (CO) poisoning according to (A) sex and (B) standardized incidence ratios (SIR) (100 000 person/year) of newly diagnosed stroke by age groups. The incidence of CO poisoning was higher in males than in females, and the numbers of patients with CO poisoning peaked in those aged 30–39 years. Although SIRs tended to increase with age, the observed number of patients who developed stroke after CO poisoning was higher in those aged ≤54 years than those aged ≥75 years.
Figure 2. Overall distribution of patients with carbon monoxide (CO) poisoning according to (A) sex and (B) standardized incidence ratios (SIR) (100 000 person/year) of newly diagnosed stroke by age groups. The incidence of CO poisoning was higher in males than in females, and the numbers of patients with CO poisoning peaked in those aged 30–39 years. Although SIRs tended to increase with age, the observed number of patients who developed stroke after CO poisoning was higher in those aged ≤54 years than those aged ≥75 years. 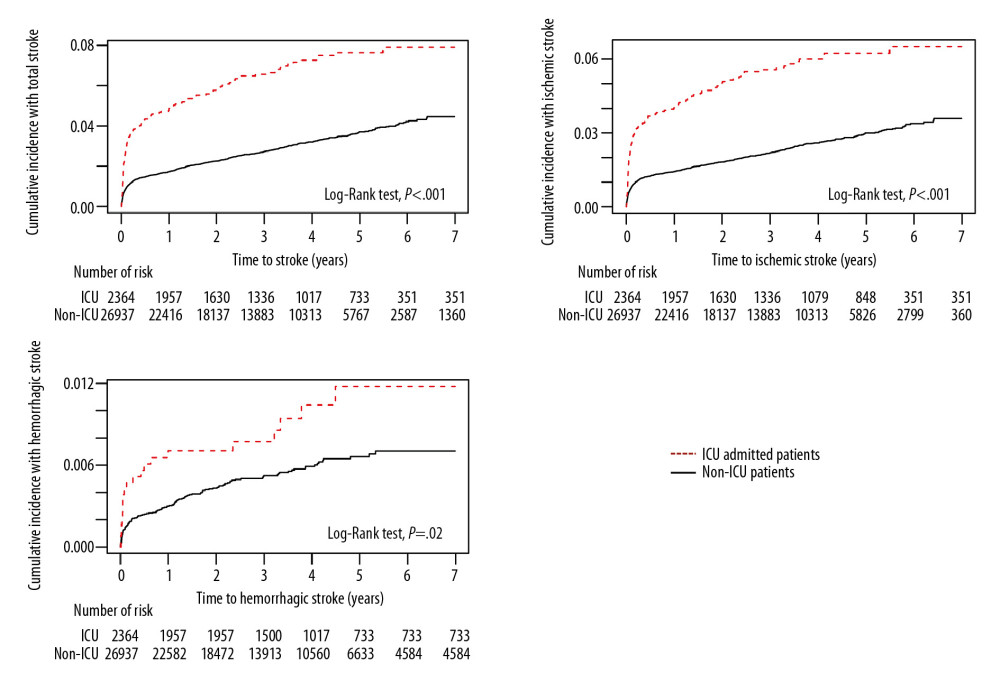 Figure 3. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide (CO) poisoning in patients who were and were not admitted to the Intensive Care Unit (ICU) (X-axis: follow-up time in years). The cumulative hazard ratio for the incidence of all types of stroke after CO poisoning was significantly higher in patients who were than were not admitted to the ICU at initial hospital visit for CO poisoning.
Figure 3. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide (CO) poisoning in patients who were and were not admitted to the Intensive Care Unit (ICU) (X-axis: follow-up time in years). The cumulative hazard ratio for the incidence of all types of stroke after CO poisoning was significantly higher in patients who were than were not admitted to the ICU at initial hospital visit for CO poisoning. 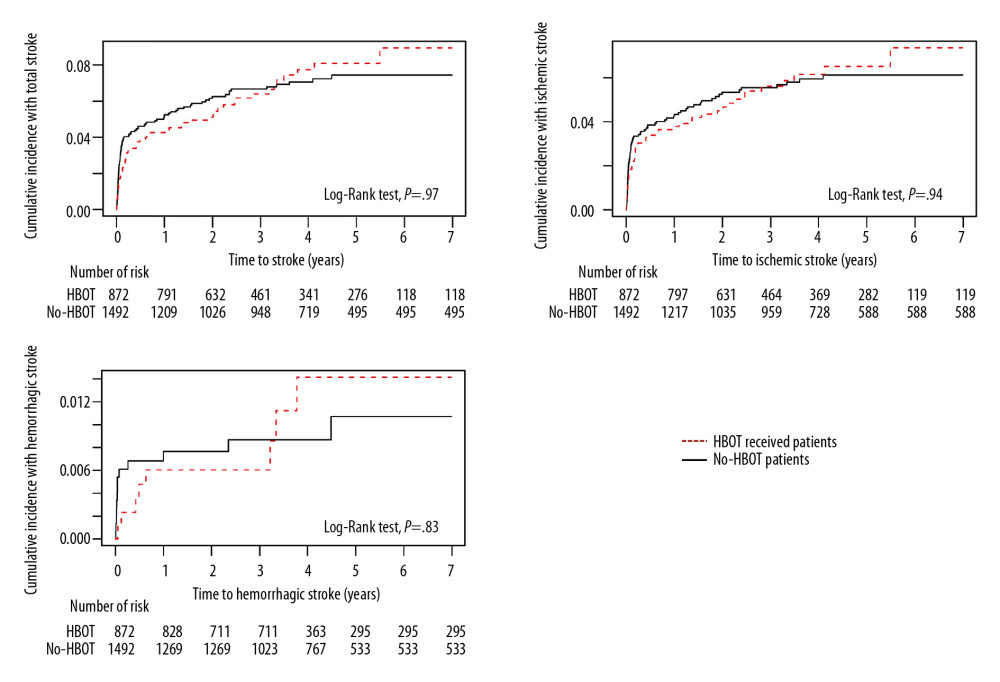 Figure 4. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide poisoning in patients who did and did not receive hyperbaric oxygen therapy (HBOT) (X-axis: follow-up time in years). The cumulative hazard rates for all types of stroke did not differ significantly in groups that did and did not receive HBOT.
Figure 4. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide poisoning in patients who did and did not receive hyperbaric oxygen therapy (HBOT) (X-axis: follow-up time in years). The cumulative hazard rates for all types of stroke did not differ significantly in groups that did and did not receive HBOT. Tables
Table 1. Baseline characteristics of total enrolled patients (total number=29 301).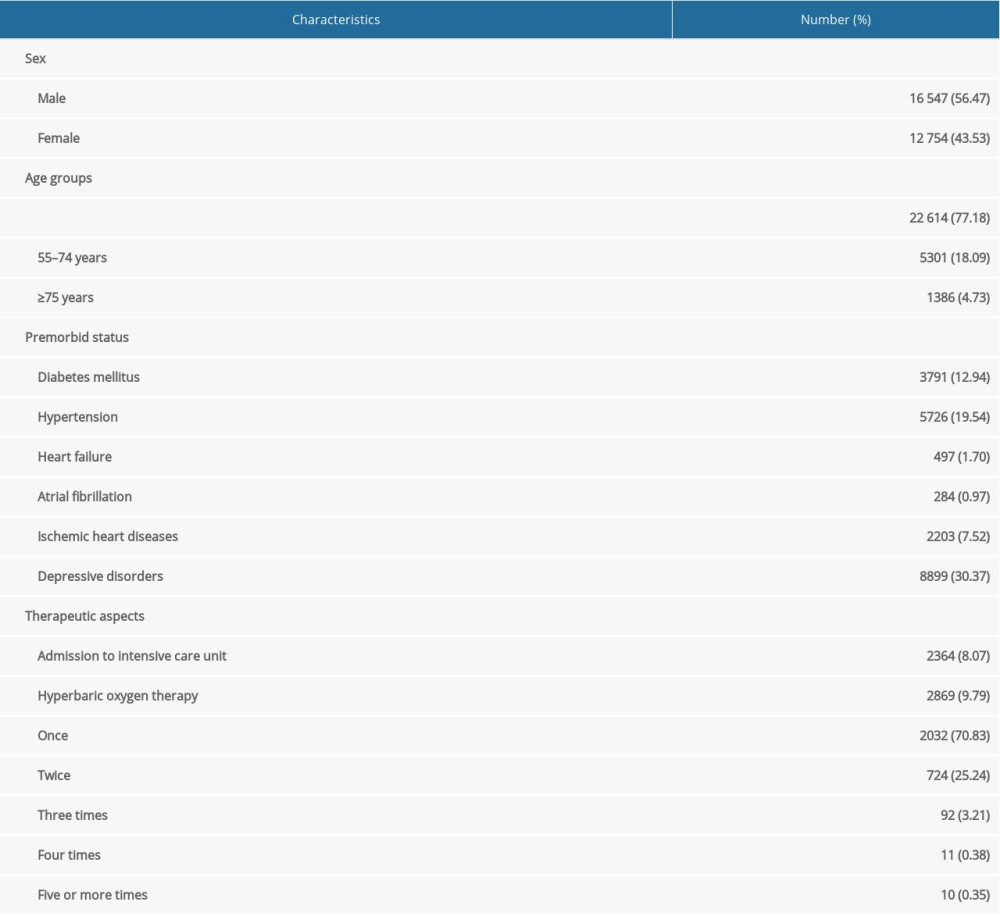 Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning.
Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning.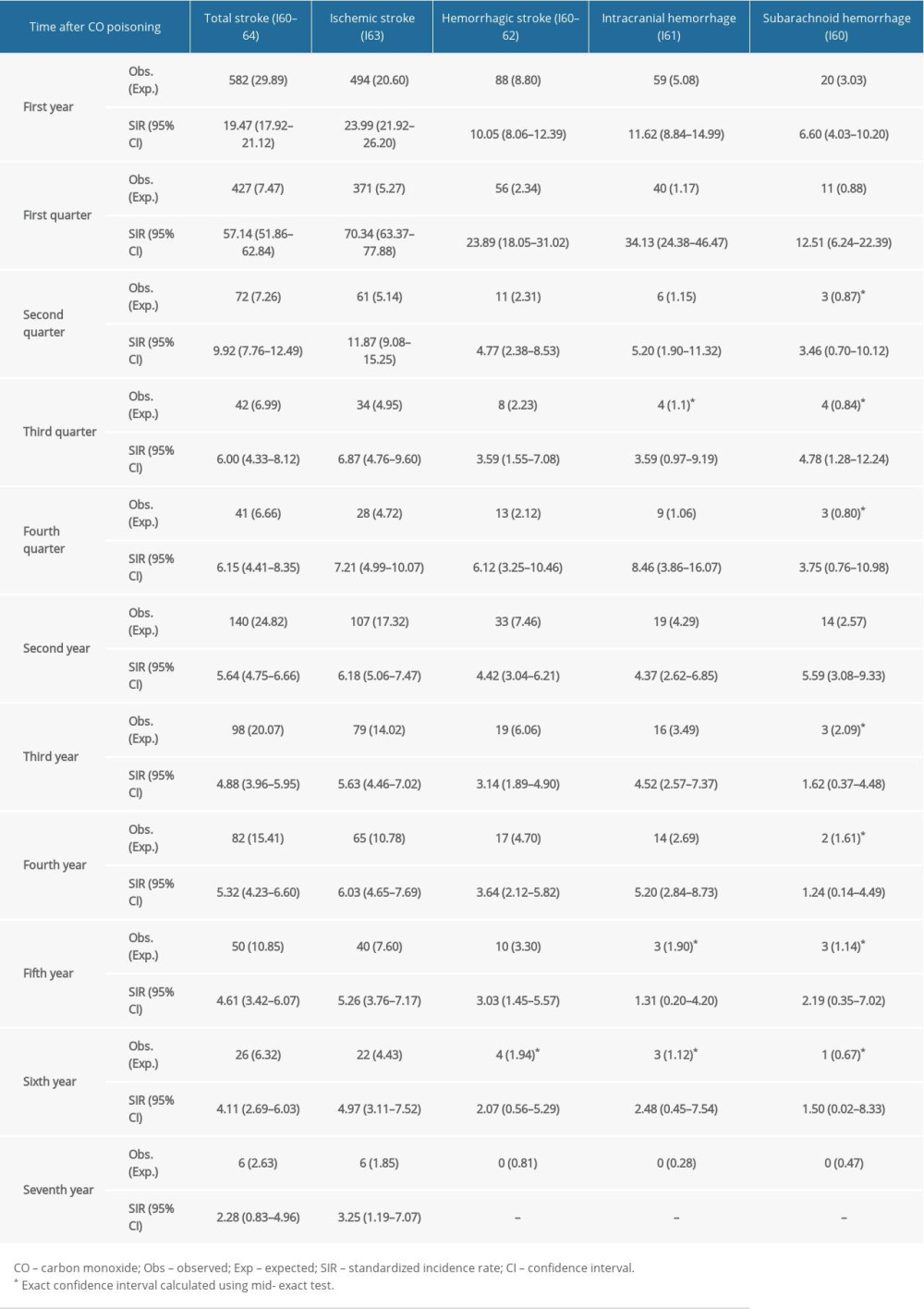 Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea.
Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea.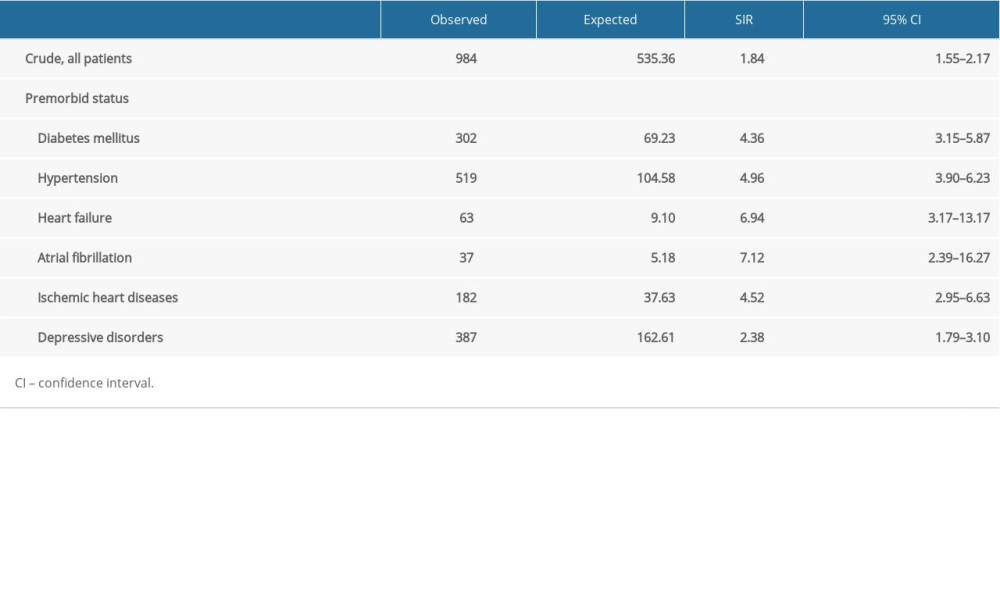
References
1. Mozaffarian D, Benjamin EJ, Go ASWriting Group Members, Heart disease and stroke statistics-2016 update: A report From the American Heart Association: Circulation, 2016; 133(4); e38-360
2. Kim JY, Kang K, Kang J, Executive summary of stroke statistics in Korea 2018: A report from the Epidemiology Research Council of the Korean Stroke Society: J Stroke, 2019; 21(1); 42-59
3. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015: Lancet, 2016; 388(10053); 1545-602
4. Donnan GA, Fisher M, Macleod M, Davis SM, Stroke: Lancet, 2008; 371(9624); 1612-23
5. Della-Morte D, Guadagni F, Palmirotta R, Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments: Pharmacogenomics, 2012; 13(5); 595-613
6. O’Donnell MJ, Xavier D, Liu L, Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study: Lancet, 2010; 376(9735); 112-23
7. Romero JR, Preis SR, Beiser A, Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study: Stroke, 2014; 45(5); 1492-94
8. Ryu JA, Bang OY, Suh GY, Ischemic stroke in critically ill patients with malignancy: PLoS One, 2016; 11(1); e0146836
9. Ng JLW, Chan MTV, Gelb AW, Perioperative stroke in noncardiac, nonneurosurgical surgery: Anesthesiology, 2011; 115(4); 879-90
10. Bushnell C, McCullough LD, Awad IA, Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2014; 45(5); 1545-88
11. Hills NK, Johnston SC, Sidney S, Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke: Ann Neurol, 2012; 72(6); 850-58
12. Oh S, Choi SC, Acute carbon monoxide poisoning and delayed neurological sequelae: A potential neuroprotection bundle therapy: Neural Regen Res, 2015; 10(1); 36-38
13. Oh BJ, Im YG, Park E, Treatment of acute carbon monoxide poisoning with induced hypothermia: Clin Exp Emerg Med, 2016; 3(2); 100-4
14. Beppu T, The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: A review of the literature: Am J Neuroradiol, 2014; 35(4); 625-31
15. Lin CW, Chen WK, Hung DZ, Association between ischemic stroke and carbon monoxide poisoning: A population-based retrospective cohort analysis: Eur J Intern Med, 2016; 29; 65-70
16. Ahmad A, Sharma VK, Early subcortical ischemic infarction and delayed leucoencephalopathy after carbon monoxide poisoning: Intern Emerg Med, 2012; 7(Suppl 1); S33-34
17. Bayramoglu A, Kocak AO, Kadioglu E, Ischemic stroke due to carbon monoxide intoxication: Two case reports: World J Emerg Med, 2018; 9(1); 73-75
18. Kim JA, Yoon S, Kim LY, Kim DS, Towards actualizing the value potential of Korea Health Insurance Review ans Assessment (HIRA) data as a resource for health resaerch: Strengths, limitations, applications, and strategies for optimal use of HIRA data: J Korean Med Sci, 2017; 32(5); 718-28
19. Jung KW, Won YJ, Oh CMCommunity of Population-Based Regional Cancer Registries, Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2014: Cancer Res Treat, 2017; 49(2); 292-305
20. Kim HH, Choi SC, Ahn JH, Analysis of trends in usage of analgesics and sedatives in intensive care units of South Korea: A retrospective nationwide population-based study: Medicine (Baltimore), 2018; 97(35); e12126
21. Cho Y, Kang H, Oh J, Risk of venous thromboembolism after carbon monoxide poisoning: A nationwide population-based study: Ann Emerg Med, 2020; 75(5); 587-96
22. Roquer J, Segura T, Serena J, Castillo J, Endothelial dysfunction, vascular disease and stroke: The ARTICO study: Cerebrovasc Dis, 2009; 27(Suppl 1); 25-37
23. Parfenova H, Basuroy S, Bhattacharya S, Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: Contributions of HO-1 and HO-2 to cytoprotection: Am J Physiol Cell Physiol, 2006; 290(5); C1399-410
24. Jung YS, Lee JS, Min YG, Carbon monoxide-induced cardiomyopathy: Circ J, 2014; 78(6); 1437-44
25. Gedela M, Weltman NY, Chavvakula NS, Atrial fibrillation induced by carbon monoxide poisoning and successful treatment with hyperbaric oxygen: S D Med, 2017; 70(7); 319-21
26. Gursoy-Ozdemir Y, Can A, Dalkara T, Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia: Stroke, 2004; 35(6); 1449-53
27. Kim HH, Choi S, Therapeutic aspects of carbon monoxide in cardiovascular disease: Int J Mol Sci, 2018; 19(8); 2381
Figures
 Figure 1. (A) Research design of this retrospective cohort study. Patients diagnosed with CO poisoning from January 1, 2012, to December 31, 2018, were followed up to determine the occurrence of stroke. (B) Patient selection process. Of the 29 301 patients diagnosed with CO poisoning from 2012 to 2018, 984 (3.36%) were subsequently diagnosed with stroke. (C) Frequency of stroke after CO poisoning in survivors. Most strokes occurred during the first 2 years after CO poisoning.
Figure 1. (A) Research design of this retrospective cohort study. Patients diagnosed with CO poisoning from January 1, 2012, to December 31, 2018, were followed up to determine the occurrence of stroke. (B) Patient selection process. Of the 29 301 patients diagnosed with CO poisoning from 2012 to 2018, 984 (3.36%) were subsequently diagnosed with stroke. (C) Frequency of stroke after CO poisoning in survivors. Most strokes occurred during the first 2 years after CO poisoning. Figure 2. Overall distribution of patients with carbon monoxide (CO) poisoning according to (A) sex and (B) standardized incidence ratios (SIR) (100 000 person/year) of newly diagnosed stroke by age groups. The incidence of CO poisoning was higher in males than in females, and the numbers of patients with CO poisoning peaked in those aged 30–39 years. Although SIRs tended to increase with age, the observed number of patients who developed stroke after CO poisoning was higher in those aged ≤54 years than those aged ≥75 years.
Figure 2. Overall distribution of patients with carbon monoxide (CO) poisoning according to (A) sex and (B) standardized incidence ratios (SIR) (100 000 person/year) of newly diagnosed stroke by age groups. The incidence of CO poisoning was higher in males than in females, and the numbers of patients with CO poisoning peaked in those aged 30–39 years. Although SIRs tended to increase with age, the observed number of patients who developed stroke after CO poisoning was higher in those aged ≤54 years than those aged ≥75 years. Figure 3. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide (CO) poisoning in patients who were and were not admitted to the Intensive Care Unit (ICU) (X-axis: follow-up time in years). The cumulative hazard ratio for the incidence of all types of stroke after CO poisoning was significantly higher in patients who were than were not admitted to the ICU at initial hospital visit for CO poisoning.
Figure 3. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide (CO) poisoning in patients who were and were not admitted to the Intensive Care Unit (ICU) (X-axis: follow-up time in years). The cumulative hazard ratio for the incidence of all types of stroke after CO poisoning was significantly higher in patients who were than were not admitted to the ICU at initial hospital visit for CO poisoning. Figure 4. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide poisoning in patients who did and did not receive hyperbaric oxygen therapy (HBOT) (X-axis: follow-up time in years). The cumulative hazard rates for all types of stroke did not differ significantly in groups that did and did not receive HBOT.
Figure 4. Kaplan-Meier analysis of the cumulative incidence of stroke after carbon monoxide poisoning in patients who did and did not receive hyperbaric oxygen therapy (HBOT) (X-axis: follow-up time in years). The cumulative hazard rates for all types of stroke did not differ significantly in groups that did and did not receive HBOT. Tables
 Table 1. Baseline characteristics of total enrolled patients (total number=29 301).
Table 1. Baseline characteristics of total enrolled patients (total number=29 301). Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning.
Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning. Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea.
Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea. Table 1. Baseline characteristics of total enrolled patients (total number=29 301).
Table 1. Baseline characteristics of total enrolled patients (total number=29 301). Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning.
Table 2. Serial annual incidence rate of stroke types after carbon monoxide (CO) poisoning. Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea.
Table 3. Sub-analysis of standardized incidence ratios (SIR) for stroke following carbon monoxide (CO) poisoning according to premorbid status from 2012 to 2018 in South Korea. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








