23 October 2022: Articles 
A 60-Year-Old Woman with Primary Biliary Cholangitis and Crohn’s Ileitis Following the Suspension of Ursodeoxycholic Acid
Unusual clinical course, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Mario Romeo- A Study design/planning
- B Data collection/entry
- D Data interpretation
- E Preparation of manuscript
- F Literature analysis/search
- A Study design/planning
- B Data collection/entry
- E Preparation of manuscript
- F Literature analysis/search
- A Study design/planning
- E Preparation of manuscript
- B Data collection/entry
- E Preparation of manuscript
- F Literature analysis/search
- B Data collection/entry
- D Data interpretation
- B Data collection/entry
- D Data interpretation
- B Data collection/entry
- D Data interpretation
- A Study design/planning
- C Data analysis/statistics
- E Preparation of manuscript
- F Literature analysis/search
- *Corresponding author: [email protected]
- A Study design/planning
- B Data collection/entry
- D Data interpretation
DOI: 10.12659/AJCR.936387
Am J Case Rep 2022; 23:e936387
Abstract
BACKGROUND: There is a recognized association between inflammatory bowel disease (IBD) and hepatobiliary autoimmune disease, particularly primary sclerosing cholangitis (PSC). There have been fewer reported cases of IBD and primary biliary cholangitis (PBC), which is treated with ursodeoxycholic acid (UDCA). This report presents the case of a 60-year-old woman with PBC who was diagnosed with Crohn’s ileitis after suspension of UDCA treatment.
CASE REPORT: A 66-year-old female patient with PBC was admitted to our department for irrepressible chronic diarrhea and recurrent abdominal pain. PBC was diagnosed on the basis of serological data: chronic (>6 months) increase in alkaline phosphatase (ALP) associated with positivity for specific anti-nuclear antibodies (sp100 and gp210), without requiring a liver biopsy and a magnetic resonance cholangiopancreatography to rule out PSC. Given the intolerance and non-responsiveness according to the Toronto criteria (ALP <1.67 times the normal limit after 2 years) to UDCA at 15 mg/kg/day, an oral monotherapy treatment using obeticholic acid at 5 mg/day was prescribed. The patient complained of abdominal pain and upper gastrointestinal symptoms. The endoscopic/histologic and radiologic examinations supported the diagnosis of Crohn’s ileitis. Given the potential benefits to PBC patients of what is described as off-label therapy, budesonide at a dosage of 9 mg/day p.o. was also administered. One month after discharge, an improvement was observed both in the cholestasis indices and in gastrointestinal symptoms.
CONCLUSIONS: This report presents a case of PBC in which the patient was diagnosed with Crohn’s ileitis after cessation of treatment with UDCA, and highlights the importance of recognizing the association between autoimmune hepatobiliary disease and IBD.
Keywords: Crohn Disease, Inflammatory Bowel Diseases, Liver Cirrhosis, Biliary, Female, Humans, Aged, Middle Aged, ursodeoxycholic acid, Alkaline Phosphatase, Autoimmune Diseases, Budesonide, Abdominal Pain, Ileitis
Background
Primary biliary cholangitis (PBC) is a rare chronic inflammatory cholestatic autoimmune liver disease, potentially culminating in end-stage biliary cirrhosis in the absence of adequate treatment [1]. Epidemiologically, this condition represents a female-predominant disease with a typical age of onset of greater than 40 years [2]. The clinical picture of PBC is extremely varied, including common (eg pruritus, abdominal discomfort, sicca complex), possible (eg jaundice, fatigue, restless legs), and rare (eg neurological, including insomnia, depression, and cognitive dysfunction) manifestations [1]. However, an asymptomatic or nonspecific chronic course more often occurs, which delays, unavoidably, the activation of the appropriate diagnostic workup. Currently, PBC diagnosis can be posed in the presence of one of the following serological criteria: (i) an increase in alkaline phosphatase (ALP), maintained for at least 6 months,in the absence of other liver diseases and systemic diseases, associated with either positivity for antimitochondrial autoantibodies (AMA) (titer >1: 40) or antinuclear autoantibodies (ANA) [subtype anti-sp100 and anti-gp210], with the ANA-positive subtype also known as “AMA-negative PBC”; or (ii) a chronic increase in ALP with negative AMA and ANA tests but a liver biopsy that shows destructive cholangitis and destruction of interlobular bile ducts [1,3].
Therefore, liver biopsy is not always required, and appears crucial only when specific antibodies are absent or when coexistence of non-alcoholic steatohepatitis (NASH) or autoimmune hepatitis (AIH) is suspected [3]. The abovementioned serological findings are helpful and sufficient in the differential diagnosis between PBC and primary sclerosing cholangitis (PSC), another cholestatic autoimmune liver disease. However, when the differentiation is doubted, a magnetic resonance cholangiopancreatography (MRCP) could be performed, as MRCP is a test with high specificity and sensitivity for the detection of PSC [3,4]. Different prognostic scores considering clinical, biochemical, and histological findings have been proposed to evaluate the severity of the disease, including the Mayo score, UK-PBC score, and Global PBC Study Group (GLOBE) score [5]. Among these, the Mayo score has been extensively validated and considered the classic prognostic model for survival in untreated PBC patients [5]. Ursodeoxycholic acid (UDCA) at 13–15 mg/kg/day has hugely improved prognosis, and currently represents the first-line treatment [3,4]. The response to UDCA is evaluated on the basis of various criteria, among which the Paris II criteria (ALP level of <1.5 times the normal limit, or an AST level <1.5 times the normal limit, or a bilirubin level <1 mg/dl after one year of treatment) and Toronto criteria (ALP >1.67 times the normal limit after 2 years) represent the most used [6]. In non-UDCA-responsive or UDCA-intolerant patients (people developing adverse drug reactions such as diarrhea), the administration of obeticholic acid (OCA) has been globally approved in combination with UDCA for those with an inadequate response to UDCA, or as monotherapy in those intolerant to UDCA [1,3]. In these scenarios, OCA represents the exclusive validated second line of treatment, despite encouraging evidence regarding the usefulness of drugs such as bezafibrate and budesonide, which still remain off-label therapies for PBC [7].
Crohn’s disease is a chronic inflammatory bowel disease (IBD) clinically associated with progressive bowel damage and disability [8]. The pathogenesis remains unknown and, consequently, despite the availability of several medical agents including mesalazine, locally active steroids (budesonide), systemic steroids, thiopurines (azathioprine), methotrexate, and biological therapies, curative therapy for this condition currently has not been achieved [8]. Crohn’s disease diagnosis represents a very complex process: a complete physical examination should be performed, along with laboratory evaluations identifying inflammation markers and screening for alternative diagnoses, including the measurement of fecal calprotectin [9]. Endoscopy and radiological imaging are subsequently used to confirm the diagnosis and determine the extent of the disease [9]. Crohn’s disease prognosis can be recurrently severe and unfortunate due to the lack of a definitive cure as well as the various IBD-associated extraintestinal manifestations, which can severely impact the quality of life of patients [8].
In this sense, a recognized association between IBD and hepatobiliary autoimmune disorders, particularly PSC, has been widely described [10,11]. Although the association between IBD and PBC is not as strong as for PSC, anecdotal cases reporting the rare coexistence of IBD and PBC being treated with UDCA do exist [12,13]. In these cases, PBC has usually been identified after IBD was diagnosed [12,13].
This report presents the case of a 60-year-old woman with PBC who was diagnosed with Crohn’s ileitis after cessation of treatment with UDCA.
Case Report
A 66-year-old female patient was admitted to our department in November 2021, complaining mostly of fatigue, recurrent abdominal colic, irrepressible chronic diarrhea, and upper gastrointestinal symptoms such as nausea and postprandial distress syndrome dyspepsia.
On admission, the family history showed no clinically relevant information and was negative for both inflammatory and neo-plastic gut disorders. Regarding her past medical history, we reported Hashimoto’s thyroiditis (diagnosed about 20 years ago, currently in euthyroidism, for which she does not take any drug) and PBC (identified after the incidental finding of increased cholestatic indices, more than 5 years ago). She denied other comorbidities as well as previous and current alcohol intake. PBC was diagnosed on the basis of serological criteria: chronic (>6 months) increase of ALP associated with positivity for specific ANA (sp100 and gp210) [3]. In light of this, a liver biopsy and an MRCP to rule out PSC appeared not to be required. UDCA p.o. at 15 mg/kg/day was initially administered using the Toronto criteria (ALP <1.67 times the normal limit after 2 years) to evaluate the response to the treatment over the following 2 years. After 2 years, due both to intolerance (onset of chronic diarrhea) and non-response, the UDCA treatment was ceased. As a consequence, the patient’s chronic liver disease progressed to compensated advanced chronic liver disease (cACLD) (Child-Pugh A5). Therefore, after 3 years of UDCA wash-out, an oral monotherapy treatment using OCA at the dosage of 5 mg/day (with a re-evaluation in consideration of a possible increase in dosage to 10 mg after 6 months) was started, according to the current European Association for the Study of the Liver clinical practice guidelines [1].
Starting 1.5 years after the UDCA suspension, the patient complained of recurrent episodes of non-specific colic abdominal pain, but not bloody diarrhea, which forced her to multiple visits to the emergency room. The clinical manifestation was treated with symptom-alleviation drugs without any further medical examination to reach diagnostic conclusions that would have enabled an etiological therapeutic approach. However, she denied vomiting or episodes of sub-occlusive crisis (Figure 1).
We also collected documentation of an index endoscopic examination (colonoscopy) performed in June of 2020 highlighting a sub-stenosis of the ileocecal valve. Analysis of the histologic samples obtained from the colonoscopy demonstrated sub-atrophy of the villi and, in the lamina propria, evidence of edema, fibrosis, and vascular congestion. In addition, we observed an active, chronic, lymphoplasmacellular inflammatory infiltrate, organized in lymphofollicular and erosive aspects associated with the coexistence of various eosinophilic granulocytes.
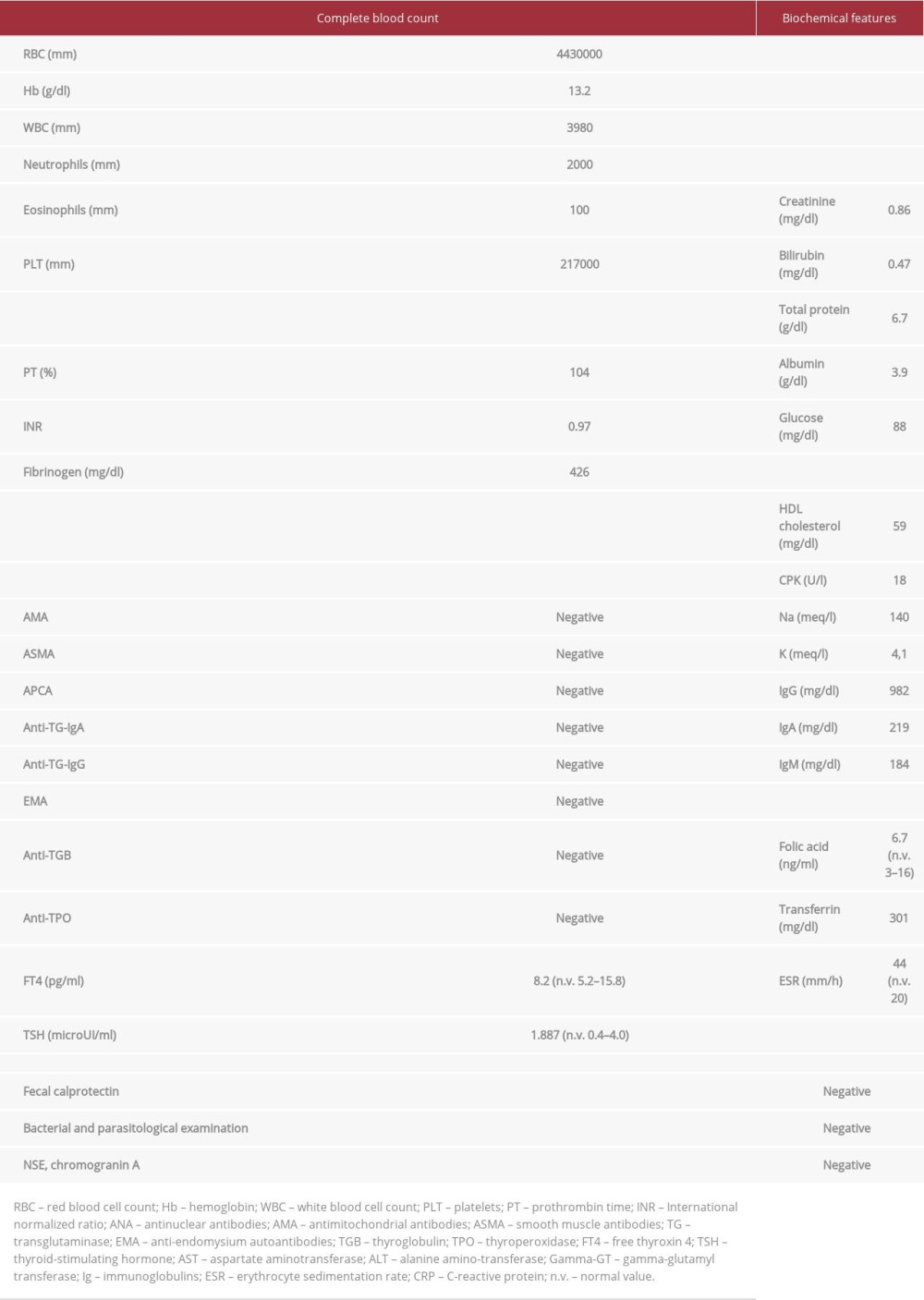
After medical history collection, a physical abdominal examination revealed that the right iliac fossa was slightly painful on palpation. In light of these data, a diagnostic workup protocol for her chronic diarrhea was performed. The results did not provide clear indications (screening for celiac disease: negative, blood eosinophil count: normal; bacterial and parasitic infection panel: negative; endocrine markers of chronic diarrhea: negative), except for the already-known increased cholestasis indices [ALP: ×3 Upper Limit Normal (ULN); gamma-glutamyl-transferase (gamma-GT): ×8 ULN] associated with sp100 and gp120 positivity, an increase in inflammation indices [erythrocyte sedimentation rate: ×2 ULN; C-reactive protein (CRP): ×6 ULN], and a vitamin B12 deficiency. Laboratory investigation results are fully reported in Table 1.
Next, endoscopic examinations were performed. Esophagogastroduodenoscopy revealed a picture compatible with “
Pancolonscopy confirmed an ileal sub-stenosis: “
An MRI enterography was also performed: “
By combining the clinical [Crohn’s Disease Activity Index: 215 points], endoscopic [Simple Endoscopic Score for Crohn’s Disease: 3], and radiologic (London Score >4.1) pictures, the final diagnosis of active Crohn’s ileitis causing ileal sub-stenosis in a patient with concomitant PBC was made [15–17]. Given the concomitance of ileal Crohn’s disease and the previously described apparent benefits of off-label therapy for PBC patients [1,3,18], budesonide at a dosage of 9 mg/day p.o. was prescribed.
One month after discharge, at the outpatient check-up, a clear improvement was observed in the cholestasis indices [ALP: <×2 ULN and gamma-GT: ×3 ULN], inflammation indices (CRP: ×2 ULN), and intestinal symptoms (especially in terms of diarrheal and abdominal pain control), revealing an initial success of the combined treatment.
Considering the well-known hepatotoxicity of budesonide in the long term [1,7] as well as the not-excludable possibility of ileal sub-stenosis necessitating a further surgical approach, the patient will need close followup. Therefore, we planned a trimestral followup schedule to manage her biochemical and clinical conditions, adopting a multidisciplinary (hepatologists and IBD experts) management team, which was available in our department.
Discussion
The reported case presents some interesting epidemiological and clinical features offering food for thought on the management of coexisting Crohn’s disease and PBC. The patient was a 66-year-old woman for whom the diagnosis of Crohn’s disease followed the cessation of treatment with UDCA previously administered for PBC. As she was unable to tolerate the UDCA, to which her PBC was also non-responsive, it was required that completely new and unexplored diagnostic and therapeutic possibilities be considered.
IBD is frequently associated with different extraintestinal manifestations, including various hepatobiliary disorders [19]. It is not clear whether the concomitance of these conditions is accidental or explainable in light of common pathogenetic traits. Corroborating the latter explanation, in the scientific literature, some evidence indicates that these conditions share association with common genetic variants, particularly regarding genes involved in the regulation of immune reactance [20–22]. On the other hand, a large Polish population study has contradicted these data, placing strong doubts on the theory of common genetic background [23]. In our case, the existence of a common denominator linking the diseases based on immune-genetic mechanisms appears reinforced by the concomitant presence in the same patient of an adjunctive autoimmune disease (in addition to her PBC and Crohn’s disease): Hashimoto thyroiditis. Thyroid dysfunction is frequently associated with both PBC and Crohn’s disease, sometimes preceding the diagnosis of both diseases [24]. Nevertheless, some points concerning the diagnosis and clinical outcome of coexisting PBC and Crohn’s disease have to be discussed. Firstly, it is important to consider that, in reporting that “in our patient the onset of PBC occurred before Crohn’s disease diagnosis,” a point of weakness could exist. On the one hand, she did not present alterations of the alvus or other symptoms suggestive of Crohn’s disease before the diagnosis of PBC. On the other hand, IBD might have already been present in a clinically and biochemically silent form. However, the assumption that PBC preceded the onset of Crohn’s disease in this patient remains until proven otherwise, since she had never undergone endoscopic examinations with biopsies before being diagnosed with PBC, even if we have to consider the large lapse of time dividing the occurrence of PBC and Crohn’s disease.
The second point necessary to be discussed is the correct order of medical events that appear to be relevant to early determination of optimal clinical management. Towards this end, 2 possible scenarios are plausible. In the first scenario, the diagnosis of PBC occurs after a long period of Crohn’s disease. In this case, the patient may be a candidate who could benefit from administration of anti-TNF-alpha drugs used for IBD, such as adalimumab or infliximab [8]. In the second scenario, in contrast, the Crohn’s disease diagnosis is concomitant or follows that of PBC. In this case, it is likely that the patient has never experienced other drugs for IBD (local or systemic steroids) and/or the disease severity has not reached the point of justification of biological therapies based on anti-TNF-alpha molecules [8].
In the first situation, the drug may be efficacious in treating PBC, as has been described anecdotally in a reported case in which adalimumab resulted in effective control of Hashimoto thyroiditis, Crohn’s disease, and PBC in the same patient [25].
Obviously, in this case, PBC-specific treatment based on UDCA (and in the case of failure or intolerance of OCA) must be continued [7]. In the second case, the choice of the most appropriate course of management appears very complex, considering also that there is no evidence justifying the routine clinical use of anti-TNF-alpha as an available drug for PBC patients [7]. The latter appears as the scenario that better reflects the clinical case reported above.
Based on these considerations, therefore, being unable to administer anti-TNF-alpha drugs (both because they are not approved for PBC and because they are not justified by the clinical history of Crohn’s disease [8] in the present case report), the best therapeutic strategy appeared to be to treat the PBC with OCA and the Crohn’s disease with budesonide. This would also serve to exploit the effects that this steroid can also have on hepatobiliary disease [1,7]. In this regard, a recent placebo-controlled randomized trial on the use of budesonide for PBC following an insufficient response to UDCA demonstrated that budesonide add-on therapy was associated with improvements in biochemical markers of disease activity but no improvement in liver histology [18]. However, careful monitoring needed to be carried out in this patient because budesonide is metabolized for the most part by the liver, and therefore, drug-induced liver injury can sometimes occur [1,7]. In addition, ileal sub-stenosis, if asymptomatic and without a history of subocclusive crises, may not benefit from pharmacological treatment alone and may eventually require a surgical approach.
The clinical scenario of co-occurring IBD and hepatobiliary disorders differs from that of PSC, which is often associated with IBD, especially ulcerative colitis. The reported cases of concomitant PBC and Crohn’s disease are extremely rare, and existing reports on the coexistence of these 2 conditions typically describe younger patients diagnosed with PBC subsequently to, or concomitantly with, Crohn’s disease [12,13,26].
Liberal et al reported a series of 6 patients (including 5 women whose ages ranged from 22.5 to 48.8) with PBC-associated IBD, of which 3 had Crohn’s disease and 3 had ulcerative colitis. However, in contrast to our case, in which diagnosis of Crohn’s disease occurred after the onset of AMA-negative, UDCA non-responsive PBC, in these abovementioned series, PBC was diagnosed in all of the patients after IBD had been diagnosed. In addition, all of the patients were positive for AMA antibodies, and 1 year after initiation of UDCA, all of the patients responded to therapy [12]. Similarly, other cases of co-occurring Crohn’s disease and PBC have been AMA seropositive forms of the hepatobiliary disorder, and, once again, a favorable response to UDCA treatment was seen, as evaluated using Paris II criteria [13].
We report here the case of a 66-year-old female patient, not affected by other comorbidities except for Hashimoto’s thyroiditis, in which the onset of AMA-negative PBC preceded ileal Crohn’s disease diagnosis. PBC was diagnosed serologically more than 5 years previous to the Crohn’s disease diagnosis and was initially treated using UDCA. Unfortunately, after 2 years, the patient had to stop UDCA because of both intolerance (severe diarrhea) and non-responsiveness (according Toronto criteria) [1], facilitating the progression of the disease up to compensated advanced chronic liver disease development.
However, despite the period of UDCA wash-out, diarrheic episodes persisted as well as “upper” gastrointestinal problems (nausea and postprandial distress syndrome dyspepsia), which were associated with worsening fatigue. While the nausea and postprandial distress syndrome dyspepsia could be explained by her autoimmune hepatobiliary disorder, the intractable diarrhea wasn’t completely attributable to PBC, especially considering the recurrence of abdominal colic pain episodes. The examinations performed during hospitalization in our department showed the presence of an ileal sub-stenosis with evidence of a histologic sub-acute inflammatory process, compatible with ileal Crohn’s disease diagnosis. Therefore, oral administration of budesonide 9 mg/day was started, toward the goal of gaining benefits in treating both PBC and Crohn’s disease, through a symbiotic therapeutic effect.
One month after discharge from the hospital, an improvement in cholestasis indices and severity of gastrointestinal symptoms was observed. This confirmed, at least for the moment, the efficacy of the treatment for both diseases. However, considering the hepatotoxicity of budesonide [1,7], as well as the possibility that the ileal sub-stenosis might require surgery, we plan to strictly follow the patient, via a multidisciplinary approach involving hepatologists and IBD experts. Indeed, despite the biochemical and clinical benefits obtained after a month of combined treatment, the case continues to be very complex and the question of whether to consider (and thus treat) the 2 coexisting pathologies as “distinct” or “in parallel” still remains.
In this sense, the best therapeutic strategy dictates that a multidisciplinary approach with close followup over the next few months is crucial.
Conclusions
This report presents a case of PBC in a patient who was diagnosed with Crohn’s ileitis after cessation of treatment with UDCA, and highlights the importance of recognizing the association between autoimmune hepatobiliary disease and IBD. Even though this presentation is considered atypical and rare, according to our reported experience, all patients with IBD should be screened not only for PSC but also for other hepatobiliary manifestations, such as PBC, and vice-versa. This could facilitate and optimize the management of both diseases through the early setting of a tailored and adequate therapeutic path.
Figures
References:
1.. , EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis: J Hepatol, 2017; 67(1); 145-72
2.. Tanaka A, Current understanding of primary biliary cholangitis: Clin Mol Hepatol, 2021; 27(1); 1-21
3.. Younossi ZM, Bernstein D, Shiffman ML, Diagnosis and management of primary biliary cholangitis: Am J Gastroenterol, 2019; 114(1); 48-63
4.. Khoshpouri P, Habibabadi RR, Hazhirkarzar B, Imaging features of primary sclerosing cholangitis: From diagnosis to liver transplant follow-up: Radiographics, 2019; 39(7); 1938-64
5.. Goet JC, Murillo Perez CF, Harms MH, A Comparison of prognostic scores (Mayo, UK-PBC, and GLOBE) in primary biliary cholangitis: Am J Gastroenterol, 2021; 116(7); 1514-22
6.. Parés A, Caballería L, Rodés J, Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid: Gastroenterology, 2006; 130(3); 715-20
7.. Cazzagon N, Floreani A, Primary biliary cholangitis: treatment: Curr Opin Gastroenterol, 2021; 37(2); 99-104
8.. Torres J, Bonovas S, Doherty G, ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment: J Crohns Colitis, 2020; 14(1); 4-22
9.. Veauthier B, Hornecker JR, Crohn’s disease: Diagnosis and management: Am Fam Physician, 2018; 98(11); 661-69
10.. Loftus EV, Harewood GC, Loftus CG, PSC-IBD: A unique form of inflammatory bowel disease associated with primary sclerosing cholangitis: Gut, 2005; 54(1); 91-96
11.. Palmela C, Peerani F, Castaneda D, Inflammatory bowel disease and primary sclerosing cholangitis: A review of the phenotype and associated specific features: Gut Liver, 2018; 12(1); 17-29
12.. Liberal R, Gaspar R, Lopes S, Macedo G, Primary biliary cholangitis in patients with inflammatory bowel disease: Clin Res Hepatol Gastroenterol, 2020; 44(1); e5-e9
13.. Efe C, Torgutalp M, Henriksson I, Extrahepatic autoimmune diseases in primary biliary cholangitis: Prevalence and significance for clinical presentation and disease outcome: J Gastroenterol Hepatol, 2021; 36(4); 936-42
14.. Knox C, Almeida J, The comb sign: Clin Gastroenterol Hepatol, 2021; 19(10); A29-30
15.. Yoshida EM, The Crohn’s Disease Activity Index, its derivatives and the Inflammatory Bowel Disease Questionnaire: A review of instruments to assess Crohn’s disease: Can J Gastroenterol, 1999; 13(1); 65-73
16.. D’Amico F, Chateau T, Laurent V, Which MRI Score and technique should be used for assessing Crohn’s disease activity?: J Clin Med, 2020; 9(6); 1691
17.. Daperno M, D’Haens G, Van Assche G, Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SESCD: Gastrointest Endosc, 2004; 60(4); 505-12
18.. Hirschfield GM, Beuers U, Kupcinskas L, A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA: J Hepatol, 2021; 74(2); 321-29
19.. Harbord M, Annese V, Vavricka SR, The First European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease: J Crohns Colitis, 2016; 10(3); 239-54
20.. Sun Y, Irwanto A, Toyo-Oka L, Fine-mapping analysis revealed complex pleiotropic effect and tissue-specific regulatory mechanism of TNFSF15 in primary biliary cholangitis, Crohn’s disease and leprosy: Sci Rep, 2016; 6; 31429
21.. Aiba Y, Yamazaki K, Nishida N, Disease susceptibility genes shared by primary biliary cirrhosis and Crohn’s disease in the Japanese population: J Hum Genet, 2015; 60(9); 525-31
22.. Gravina AG, Dallio M, Masarone M, Vascular endothelial dysfunction in inflammatory bowel diseases: Pharmacological and nonpharmacological targets: Oxid Med Cell Longev, 2018; 2018; 2568569
23.. Gaj P, Habior A, Mikula M, Ostrowski J, Lack of evidence for association of primary sclerosing cholangitis and primary biliary cirrhosis with risk alleles for Crohn’s disease in Polish patients: BMC Med Genet, 2008; 9; 81
24.. Crowe JP, Christensen E, Butler J, Primary biliary cirrhosis: the prevalence of hypothyroidism and its relationship to thyroid autoantibodies and sicca syndrome: Gastroenterology, 1980; 78(6); 1437-41
25.. Triantafillidis JK, Durakis S, Merikas E, Crohn’s disease of the small bowel, complicated by primary biliary cirrhosis, Hashimoto thyroiditis, and Raynaud’s phenomenon: Favorable response of all disorders to adalimumab treatment: Gastroenterol Hepatol Bed Bench, 2013; 6(2); 101-5
26.. Gizard E, Ford AC, Bronowicki JP, Peyrin-Biroulet L, Systematic review: The epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease: Aliment Pharmacol Ther, 2014; 40(1); 3-15
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250