14 May 2022: Articles 
Case Series of Successful Intravenous Immunoglobulin (IVIG) Treatment in 4 Pregnant Patients with Severe COVID-19-Induced Hypoxia
Unusual or unexpected effect of treatment, Rare disease
Matthew Geriak1ABCDEF*, Dominic McGrosso23DEF, David J. Gonzalez23DEF, Matthew Dehner4AD, George Sakoulas156ADEFGDOI: 10.12659/AJCR.936734
Am J Case Rep 2022; 23:e936734
Abstract
BACKGROUND: Despite unprecedented speed in the execution of the COVID-19 vaccine and therapeutic clinical trials, pregnant patients have been largely excluded from initial studies. In addition, pregnant patients who are unvaccinated against SARS-CoV-2 have greater morbidity risk with severe COVID-19 disease as compared to patients of similar age and comorbidity status. Intravenous immunoglobulin (IVIG) has been deemed safe in pregnancy in other diseases. Prior data demonstrate the possible benefit of utilizing IVIG for the treatment in hospitalized patients with severe respiratory symptoms associated with COVID-19 active infections when administered within 14 days of COVID symptom onset.
CASE REPORT: We administered IVIG (Privigen®, CSL Behring) 0.5 g/kg daily for 3 consecutive days to 4 pregnant patients (ages 24-34 years of age) who were hospitalized with moderate-to-severe COVID-19 and not vaccinated against SARS-CoV-2. All patients received concomitant glucocorticoid therapy. Gestational ages were 26, 17, 35, and 35 weeks. All patients were discharged home breathing room air after a mean hospital stay of 15 days. Two patients had uncomplicated cesarean section at 35 weeks during the hospitalization. The pre-term pregnancies at 17 and 26 weeks were intact at hospital discharge and resulted in normal vaginal deliveries at term. All 4 patients consented to participate in this case series report.
CONCLUSIONS: IVIG may be a safe treatment consideration in pregnant women with severe COVID-19 to avoid pregnancy complications. Its use warrants further study in pregnancy acute respiratory distress syndrome (ARDS) due to SARS-CoV-2, influenza, and other respiratory viruses to which pregnant patients are vulnerable.
Keywords: COVID-19, Immunoglobulins, Intravenous, Pregnancy, SARS-CoV-2, Adult, COVID-19, COVID-19 Vaccines, Cesarean Section, Female, Humans, Hypoxia, young adult
Background
Intravenous immunoglobulin (IVIG) has been found to have broad therapeutic applications for the treatment of a variety of inflammatory, infectious, autoimmune and viral diseases, including Kawasaki disease via multifactorial mechanisms of immune response modulation [1]. Several studies synthesized in a meta-analysis have shown benefit of IVIG treatment for COVID-19 [2].
Previous studies have shown that acute lung injury in COVID-19 may be mediated by the hyperimmune response driven by neutrophil extracellular trap (NET) formations [3,4,5]. IVIG may interfere with platelet activation, neutrophil activation and NET formation, thereby, mitigating lung injury [5,6].
Treatment options in pregnant patients with COVID-19 are limited, largely due to their traditional exclusion from early clinical trials, including those assessing COVID-19 therapeutics [7]. IVIG is not approved in the United States by the Food and Drug Administration (FDA) for the treatment of patients with severe COVID-19 hypoxia, nor is it recommended by the Centers of Disease Control (CDC) in the United States [8]. However, IVIG has been deemed safe in pregnancy as used for years to treat a variety of autoimmune conditions [9]. Here, we report our successful treatment of 4 severely- and critically ill COVID-19 pregnant patients with IVIG.
The recommended treatment strategies for symptomatic confirmed positive COVID-19 induced hypoxia requiring hospitalization has been evolving over span of the COVID-19 pandemic. As of early April 2022, the CDC’s treatment recommendations for COVID-19 confirmed positive adult hospitalized patients were remdesivir 200mg intravenous (IV) loading dose followed by remdesivir 100 mg IV daily for 4 doses starting 24 hours after the loading dose plus dexamethasone 6 mg orally or IV daily for up to 10 days [8]. As of April 4, 2022, there are no recommended treatment options for hospitalized and hypoxic COVID-19 positive pregnant patients not responding to remdesivir and dexamethasone [7,8,10,11]. The Society of Maternal Fetal Medicine (SMFM) guidelines mention that tocilizumab is not contraindicated in this special patient population, however, more data is needed before it is recommended due to the fact that the completed clinical trials have excluded pregnant subjects [7]. The SMFM guidance states that obstetrical medical providers may inquire about compassionate use of tocilizumab [7].
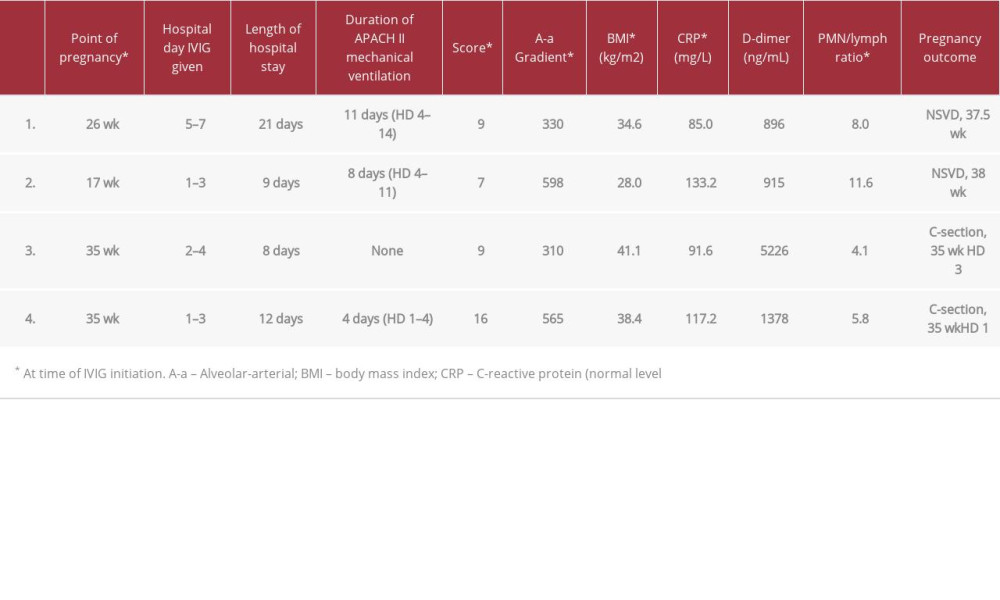
Details of the 4 cases of COVID-19 in pregnancy are provided below. All 4 cases were administered IVIG with the United States brand name, Privigen®, manufactured by CSL Behring AG. We did not utilize anti-COVID-19 IVIG (C-IVIG) that is being currently being studied in the clinical trials setting. The diagnosis of COVID-19 was confirmed by nasal swab samples sent to our hospital’s laboratory using the approved RTPCR technique. Cases 1–3 were encountered in the second COVID-19 wave in mid-2020. Case 4 was encountered in the 4th COVID-19 wave in mid-2021 when the delta SARS-CoV-2 variant accounted for the predominant burden of COVID-19 in the United States and a period during which 3 efficacious vaccines against SARS-CoV-2 were readily available. None of the patients in this case series received COVID-19 vaccinations prior to admission. Relevant clinical and laboratory details of the cases are provided in Table 1, including degree of hypoxia defined by the Alveolar-arteriolar (A-a) gradient and severity of illness by APACHE II scores at the time of IVIG administration.
A-a gradient >300 mmHg determined in a pilot study as a cut-off, whereby, IVIG administration would significantly reduce respiratory morbidity [5]. The polymorphonuclear cell/ lymphocyte ratio is provided given its prognostic significance in COVID-19, including in pregnancy [12]. This case series describes 4 pregnant women with severe/critical COVID-19 disease treated with IVIG and had good outcomes in both mother and child. In one case, rapid improvement was immediately observed after therapy.
Case Reports
CASE 1:
A 34-year-old pregnant woman (26-weeks) with a history of asthma presented to the Emergency Department (ED) with 7 days of dry cough, fever, myalgias, nausea, vomiting, and diarrhea. Her SARS-CoV-2 RT-PCR testing was positive 3 days prior to admission and confirmed COVID-19 positive by the hospital’s RT-PCR test soon after admission. A chest X-ray showed bilateral infiltrates. She was noted to be mildly hypoxemic and was admitted and placed on low-flow oxygen by nasal prongs and treated with remdesivir and dexamethasone 6 mg orally daily. Over the next 72 hours, she developed increasing respiratory distress and hypoxemia, progressing to high flow nasal cannula and ultimately placed on mechanical ventilation on hospital day 4. Her clinical condition continued to deteriorate and she became acidotic and required vasopressors. On hospital day 5, fetal heart rate monitoring showed signs of fetal distress and betamethasone was administered in anticipation of emergency cesarean section delivery. Relevant clinical and laboratory data are provided in Table 1. Starting on hospital day 5, IVIG (Privigen®) 0.5 g/kg (adjusted body weight) was administered daily for 3 days, with methylprednisolone 40 mg intravenously (IV) one hour before infusion. Within 24 hours of the first IVIG infusion, she was weaned off vasopressor support, acidosis resolved and there were no more signs of fetal distress. After 11 days of mechanical ventilation, she was extubated on hospital day 14. Oxygen was weaned off and she was discharged home on room air on hospital day 21, with no pregnancy complications. She had a normal spontaneous vaginal delivery at 37 weeks gestational age. Roughly one year post delivery, child is healthy with no complications per patient via telephone discussion. This case was also included in a prior retrospective case series of IVIG in COVID-19 [13].
CASE 2:
A 31-year-old woman pregnant at 17 weeks with no known significant medical history presented to the ED by ambulance with severe shortness of breath, dry cough, myalgia and fever for 10 days. When the Emergency Medical Service arrived at her home, she had a room air saturation 77% and respiratory rates in the 40s. She arrived at the ED on nonrebreather mask and room air blood oxygen saturation was 86%. In the ED, she was able to be converted from a nonrebreather mask to oxygen support via nasal cannula bringing the blood oxygen saturation above 93%. SARS-CoV-2 RT-PCR testing was positive 7 days prior to admission and confirmed COVID-19 positive by the hospital’s RT-PCR test soon after admission. Respiratory symptoms were escalated to severe in the ED such that she could not change position in bed without severe paroxysms of cough attacks and dyspnea. Her white count was noted to be normal with elevated transaminase levels, tachycardic with heart rate approximately 100 beats per minute and was afebrile. Due to her severe dyspnea, chest Computed Tomography (CT) angiogram was performed to rule out pulmonary embolism. This showed no pulmonary embolism, but showed multi-focal bilateral pneumonia (Figure 1A). While in the ED, her respiratory status continued to decline requiring high flow nasal cannula oxygen support and dexamethasone 10 mg IV every 12 hours was initiated. Remdesivir was not initiated due to the prolonged duration of symptoms (<7 days) prior to presentation. The patient was admitted to the COVID-19 non-ICU step-down unit. On hospital day one, IVIG (Privigen®) 0.5 g/kg (adjusted body weight) was administered daily for 3 days without methylprednisolone due to the high dose of dexamethasone. Her respiratory status failed to improve, which required escalation to respiratory support with bi-level positive airway pressure (BIPAP) and she was ultimately transferred to the ICU. On hospital day 4, placed on mechanical ventilation and transiently required vasopressor support shortly afterwards. After stabilizing on mechanical ventilation, she subsequently developed a very high fever, an increase in oxygen requirements and increased secretions. Sputum culture showed heavy growth of methicillin-susceptible Staphylococcus aureus (MSSA), placed on clindamycin 900 mg IV every 8 hours, subsequently became afebrile in 48 hours and the respiratory status started to improve. After 8 days in the ICU, she was weaned off mechanical ventilation and transferred to the floor where she completed 7 days of clindamycin and 10 days of dexamethasone. She was discharged home on hospital day 19 breathing room air and delivered vaginally at term. The child was without complications >1 year after admission per patient via telephone discussion.
CASE 3:
A 29-year-old pregnant woman at 35 weeks gestation and no known significant medical history presented to the ED with a history of fever of 7 days, positive SARS-CoV-2 RT-PCT positive test 3 days prior, worsening myalgias and dry cough. A chest X-ray showed bilateral infiltrates and she was admitted to the hospital. She declined remdesivir therapy, but accepted dexamethasone 6 mg orally daily which was initiated on hospital day 2 due to hypoxemia. IVIG (Privigen®) 0.5 g/kg (adjusted body weight) was administered daily for 3 days, also starting on hospital day 2. Of note, methylprednisolone 40 mg IV was given one hour prior to the second dose of IVIG, only because she refused for it to be given prior to dose 1 and dose 3. A cesarean section was electively performed on hospital day 3 without complications. On hospital day 4, electrocardiogram was performed revealing ST elevation in several leads. Cardiology consulted and echocardiogram was performed, which revealed dilated right ventricle and tricuspid regurgitation. On hospital day 5, CT angiography was negative (Figure 1B) for pulmonary embolism and cardiac troponin serum concentrations remained normal. She was able to be weaned off supplemental oxygen air and was comfortable breathing room air by hospital day 8. While cardiology preferred to observe she a while longer in the hospital for transient asymptomatic bradycardia, she wanted to go home and was discharged on hospital day 8. At about 1 year after delivery, the child is healthy with no complications per patient via telephone discussion.
CASE 4:
A 24-year-old pregnant woman at 35 weeks of gestation with prior history of preeclampsia during her first pregnancy presented to the ED with 5 days of fever, worsening shortness of breath and dry cough. Her SARS-CoV-2 PCR testing was positive on admission to the hospital and chest X-ray showed bilateral infiltrates consistent with COVID-19 pneumonia. She was admitted with borderline intermittent hypoxemia and treated with dexamethasone 6 mg orally daily and remdesivir. Hypoxemia quickly worsened requiring supplemental oxygen at a rate of 10 L/min via oxymizer. Also, starting on hospital day one, IVIG 0.5 g/kg (adjusted body weight) was administered daily for 3 days, with methylprednisolone 40 mg IV one hour before each infusion. Given the rapid progression throughout the first hospital day, it was decided to proceed with emergent cesarean section and due to her tenuous respiratory status, she was electively intubated and received general anesthesia for this procedure. The procedure was without complication and she was brought to the ICU afterwards for ventilator management. She remained intubated for 4 days, and was then successfully extubated and weaned to oxygen support via high flow nasal cannula. She continued on remdesivir for 5 days, dexamethasone for 10 days, along with IVIG 0.5 g/kg for 3 days. She did quite well and made fairly rapid improvement, thereafter. She started pumping breastmilk and had excellent supply per obstetrician’s chart note. Over the next week, her hypoxemia resolved and she was discharged home on hospital day 11 breathing room air without any additional supplemental oxygen. The child was healthy with no complications >1 year after admission per patient via telephone discussion.
Discussion
Guidance of COVID-19 therapy in pregnancy is extremely limited, in large part because pregnant patients are excluded from practice-evolving large randomized clinical trials. This case series is consistent with the historical safety and tolerability of IVIG use in pregnancy. Furthermore, despite severe or critical COVID-19, all 4 mother and child cases had excellent outcomes, with hospitalizations shorter than their illness severity would have predicted. One case in particular (Case 1) showed a very dramatic clinical improvement upon IVIG initiation. Pregnancy confers a relative immunosuppressed state that poses a greater risk for severe COVID-19 disease and poor maternal and fetal outcomes [14], yet therapeutics have not been studied due to exclusion of pregnant patients from early COVID clinical trials. Previous studies utilizing IVIG treatment for COVID-19 infections have given mixed results. Retrospective studies have largely been positive, yet prospective clinical trials have been mixed, with some favorable results [5,15,16] and a more recent study showing no benefit [17]. However, considerable debate still exists that some subgroups of COVID-19 patients may benefit from IVIG that are diluted by broad clinical trials which lack granularity among the heterogeneity of patient characteristics and timing of IVIG administration [18,19]. One study suggests that COVID-19 patients that may be particularly poised for IVIG benefit are those that are younger, with fewer comorbidities, and treated early [20]. Our internal drug utilization and evaluation of IVIG (Privigen®) for the treatment of severe COVID-19 patients has shown clinical and pharmacoeconomic benefit when promptly applied to such patients with rapidly deteriorating oxygenation [21]. At our institution, we targeted age <70 years, no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies) or no active treatment for cancer. The initiation of IVIG was shown to be effective if initiated within 48 hours of worsening hypoxia where oxygen requirements rapidly escalate to maintain oxygen saturation levels above 92% [21].
Pregnant patients are generally younger and have fewer comorbidities than most patients with COVID-19, but can present with high illness severity and, perhaps due to their relative immunosuppressed state, can suffer significant morbidity and mortality than comparable patients in their age group [14,22]. Intrauterine fetal demise may be mediated through placentisis, and may occur even in mild disease [23,24].
To our knowledge, there has been only one previous case published where IVIG was utilized in a pregnant patient with COVID-19. The child was delivered by C-section at 36 weeks and both mother and child survived [25]. In this case series, we present the utilization of off-label IVIG (Privigen®) in 4 pregnant patients with severe COVID-19, 3 of which became critically ill requiring mechanical ventilation. Despite very high illness severity and degree of hypoxia, all patients had excellent outcomes without any adverse consequences for them or their infants and discharged from the hospital to home in 3 weeks or less, with an average length of stay of 15 days. This duration of hospitalization is shorter than anticipated for COVID-19 patients with this degree of illness severity, particularly when they become mechanically ventilated. Once patients are admitted to the ICU, average length of stay in the ICU alone is over 12 days [26]. Two patients presented near term at 35 weeks and were delivered by cesarean section, one electively a few days after admission and one emergently within 24 hours of admission without complication. The 2 cases with second trimester pregnancies were preserved and had normal spontaneous vaginal deliveries at term at a later date. All 4 patients tolerated the IVIG therapy without any adverse drug events (including headache), without complications with the pregnancy or the COVID-19 disease state itself. All 4 patients reported having healthy children at the 1-year outcome mark. Remdesivir is offered to all patients at our institution with COVID-19 symptoms of 7 days or less. However, Case 3 declined remdesivir treatment.
The specific mechanism of how IVIG offers benefit in COVID-19 is unknown but may be in part through mitigation of endovascular NET formation. NET-mediated vascular inflammation may drive morbidity and mortality in COVID-19, just as it has been documented to do so in malaria [27] and Dengue fever [28]. Therefore, therapies that target immunothrombosis may be useful in treating respiratory failure and vascular complications in COVID-19.
We have performed early studies that are still in progress on host responses to IVIG that appear to demonstrate high levels of platelet and neutrophil degranulation markers in the blood of patients on mechanical ventilation with critical COVID-19. A reduction in platelet and neutrophil degranulation is seen following IVIG treatment, which appears related to a positive therapeutic response in COVID-19. Interestingly, baseline increases in platelet degranulation have been previously observed during pregnancy, perhaps underlying the higher risk of COVID-19 in the pregnant patient population [29].
Conclusions
In summary, we illustrate here 4 cases where IVIG was safely administered to pregnant patients with severe COVID-19 and all had excellent clinical outcomes. While there is no way of knowing whether the outcomes of the patients would have been different if compared with a placebo, IVIG may be a safe treatment consideration in pregnant women with severe COVID-19. We hope that this case series will inspire large controlled trials to be conducted with pregnant patients, not just with COVID-19, but with other viral mediated acute respiratory distress syndromes which this patient population historically has been more vulnerable to, including influenza [30].
References:
1.. Galeotti C, Kaveri SV, Bayry J, IVIG-mediated effector functions in autoimmune and inflammatory diseases: Int Immunol, 2017; 29(11); 491-98
2.. Xiang HR, Cheng X, Li Y, Luo WW, Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): A meta-analysis: Int Immunopharmacol, 2021; 96; 107732
3.. Uozumi R, Iguchi R, Masuda S, Pharmaceutical immunoglobulins reduce neutrophil extracellular trap formation and ameliorate the development of MPO-ANCA-associated vasculitis: Mod Rheumatol, 2020; 30(3); 544-50
4.. Nicolai L, Leunig A, Brambs S, Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy: Circulation, 2020; 142(12); 1176-89
5.. Sakoulas G, Geriak M, Kullar R, Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019: Crit Care Explor, 2020; 2(11); e0280
6.. Jang JE, Hidalgo A, Frenette PS, Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcγRIII and SHP-1: Circ Res, 2012; 110(8); 1057-66
7.. Halscott T, Vaught J, Management considerations for pregnant patients with COVID-19 Updated Feb. 2, 2021. Available from: https://www.smfm.org/covidclinical
8.. , Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) Updated Feb. 16, 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
9.. Han A, Lee SK, Park JC, Maternal and fetal safety of intravenous immunoglobulin in women with reproductive failure: Am J Reprod Immunol, 2021; 86(6); e13492
10.. , Coronavirus disease 2019 (COVID-19) treatment guidelines. therapeutic management of hospitalized adults with COVID-19 Updated Feb 22, 2022. Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/
11.. Bhimraj A, Morgan RL, Shumaker AH, DSA guidelines on the treatment and management of patients with COVID-19 Updated 3/23/2022. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
12.. Lasser DM, Chervenak J, Moore RM, Severity of COVID-19 respiratory complications during pregnancy are associated with degree of lymphopenia and neutrophil to lymphocyte ratio on presentation: A multicenter cohort study: Am J Perinatol, 2021; 38(12); 1236-43
13.. Herth FJF, Sakoulas G, Haddad F, Use of intravenous immunoglobulin (Prevagen or Octagam) for the treatment of COVID-19: Retrospective case series: Respiration, 2020; 99(12); 1145-53
14.. Villar J, Ariff S, Gunier RB, Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study: JAMA Pediatr, 2021; 175(8); 817-26
15.. Gharebaghi N, Nejadrahim R, Mousavi SJ, The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: A randomized placebo-controlled double-blind clinical trial: BMC Infect Dis, 2020; 20(1); 786
16.. Raman RS, Bhagwan Barge V, Anil Kumar D, A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy: J Infect Dis, 2021; 223(9); 1538-43
17.. Mazeraud A, Jamme M, Mancusi RL, Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): Multicentre, double-blind, placebo-controlled, phase 3 trial: Lancet Respir Med, 2022; 10(2); 158-66
18.. Kindgen-Milles D, Feldt T, Jensen BEO, Why the application of IVIG might be beneficial in patients with COVID-19: Lancet Respir Med, 2022; 10(2); e15
19.. Wilfong EM, Matthay MA, Intravenous immunoglobulin therapy for COVID-19 ARDS: Lancet Respir Med, 2022; 10(2); 123-25
20.. Cao W, Liu X, Hong K, High-dose intravenous immunoglobulin in severe coronavirus disease 2019: A multicenter retrospective study in China: Front Immunol, 2021; 12; 627844
21.. Poremba M, Dehner M, Perreiter A, Intravenous immunoglobulin (IVIG) in treating non-ventilated COVID-19 patients with moderate to severe hypoxia is pharmacoeconomically favorable when appropriately targeted: MedRxiv, 2021; 2021; 21264152
22.. Regan AK, Arah OA, Fell DB, Sullivan SG, SARS-CoV-2 infection during pregnancy and associated perinatal health outcomes: A National US Cohort Study: J Infect Dis, 2022; 225(5); 759-67
23.. Guan M, Johannesen E, Tang CY, Intrauterine fetal demise in the third trimester of pregnancy associated with mild infection with the SARSCoV-2 delta variant without protection from vaccination: J Infect Dis, 2022; 225(5); 748-53
24.. Shook LL, Brigida S, Regan J, SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise: J Infect Dis, 2022; 225(5); 754-58
25.. Zheng T, Guo J, He W, Coronavirus disease 2019 (COVID-19) in pregnancy: 2 case reports on maternal and neonatal outcomes in Yichang city, Hubei Province, China: Medicine (Baltimore), 2020; 99(29); e21334
26.. Vekaria B, Overton C, Wiśniowski A, Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning: BMC Infect Dis, 2021; 21(1); 700
27.. Knackstedt SL, Georgiadou A, Apel F, Neutrophil extracellular traps drive inflammatory pathogenesis in malaria: Sci Immunol, 2019; 4(40); eaaw0336
28.. Opasawatchai AO, Amornsupawat P, Jiravejchakul N, Neutrophil activation and early features of NET formation are associated with dengue virus infection in humans: Front Immunol, 2019; 9; 3007
29.. Janes SL, Goodall AH, Flow cytometric detection of circulating activated platelets and platelet hyper-responsiveness in pre-eclampsia and pregnancy: Clin Sci (Lond), 1994; 86(6); 731-39
30.. Yu W, Hu X, Cao B, Viral infections during pregnancy: The big challenge threatening maternal and fetal health: Matern Fetal Med Dec 9, 2021; 4(1); 72-86
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250