10 August 2022: Articles 
A Clinical Case of COVID-19 Vaccine-Associated Guillain-Barré Syndrome
Challenging differential diagnosis, Unexpected drug reaction, Rare disease
Alexis Hilts1EF*, Ariyon SchreiberDOI: 10.12659/AJCR.936896
Am J Case Rep 2022; 23:e936896
Abstract
BACKGROUND: Guillain-Barré syndrome (GBS) is an autoimmune condition that presents as weakness, numbness, paresthesia, and areflexia. GBS may occur following infection or vaccination. The pathogenesis of GBS is characterized by inflammatory infiltrates and segmental demyelination. The mechanism of GBS following COVID-19 vaccination is hypothesized to arise from an autoimmune-mediated mechanism leading to an increase in inflammatory cytokines. While there were no reported cases of GBS during the mRNA COVID-19 vaccination clinical trials, there have been a few case reports of GBS following COVID-19 vaccination.
CASE REPORT: We report a case of symmetric weakness and paresthesia that began 3 days after the patient received his first dose of the Moderna COVID-19 vaccine. Cerebrospinal fluid (CSF) studies demonstrated albuminocytologic dissociation. The combination of the patient’s CSF findings and clinical symptoms was concerning for Guillain-Barré syndrome. Given the clinical findings 3 days following COVID-19 vaccination, there was a high concern for COVID-19 vaccine-induced GBS. The patient was treated with IVIG followed by plasmapheresis but failed to show significant improvement from either treatment.
CONCLUSIONS: Our case report demonstrates occurrence of GBS soon after the patient received the COVID-19 Moderna vaccine. Although rare, there is some evidence to support an association between COVID-19 vaccination and GBS, but this is generally limited to case reports and case series. Clinicians, however, should remain vigilant to mitigate potential risks, such as autonomic dysfunction, respiratory failure, permanent disability, and death in patients who develop GBS after vaccination.
Keywords: Guillain-Barré syndrome, COVID-19 vaccine, COVID-19, SARS-CoV-2, Middle Aged, 2019-nCoV Vaccine mRNA-1273, COVID-19 Vaccines, Guillain-Barre Syndrome, Humans, Paresthesia
Background
COVID-19 mRNA vaccines (Pfizer-BioNTech and Moderna) have been shown to have a vaccine effectiveness rate of 82% against symptomatic illness after 1 dose and 94% after 2 doses in interim analysis [1]. These results indicate these vaccines are effective at preventing symptomatic COVID-19 disease [1]. Some patients experience adverse effects from the vaccine, while others do not experience any adverse effects [2]. Some very rare but potential adverse effects are anaphylaxis, thrombosis with thrombocytopenia syndrome, myocarditis, pericarditis, and Guillain-Barré syndrome (GBS) [2]. Although there were no reported cases of GBS during clinical trials of the mRNA COVID-19 vaccines, there have been a few case reports of GBS following COVID-19 mRNA vaccination [3,4]. The unadjusted incidence rate of GBS following mRNA vaccination was found to be 1.3 per 100 000 person-years, which is similar to the background rate of GBS [5].
GBS is an autoimmune condition characterized by numbness, bilateral weakness, and areflexia [6]. GBS can develop following infection or vaccination [7]. The mechanism is hypothesized to stem from antibodies binding to the surface of Schwann cells, leading to deposition of complement and damage to the Schwann cells [6,7]. Elevated protein in cerebrospinal fluid with normal white blood cell count, termed albuminocytologic dissociation, is characteristic of GBS [6]. The Brighton Criteria can help establish a diagnosis of GBS [8]. Treatment with plasma exchange or intravenous immune globulin are equally effective options when started within 2 weeks of symptom onset [6]. About 25% of patients with GBS develop respiratory failure; therefore, it is necessary to continually monitor respiratory function in these patients and ensure early transfer to the intensive care unit (ICU) if necessary [7]. GBS can cause dysautonomia and patients should be monitored for arrythmia, and non-ambulatory patients should receive deep vein thrombosis prophylaxis [6–7]. Unfortunately, despite immuno-therapy treatment, 4–15% of patients die and 20% of patients are disabled following GBS [6,7].
Case Report
A 58-year-old man with hypertension presented to our facility with a 4-week history of ascending symmetric weakness and paresthesia. The patient had received his first COVID-19 Moderna vaccine 3 days prior to symptom onset. Three days after receiving the vaccine, he experienced concurrent diarrhea and symmetric weakness in his lower extremities. The diarrhea resolved but the weakness persisted. The weakness was constant and prohibited him from walking or participating in activities of daily living, therefore necessitating use of a wheel-chair. Upon presentation to our facility, no work-up for the diarrheal illness was performed, as the diarrhea had resolved prior to admission and was no longer an active problem. The patient denied any history of COVID-19 prior to vaccination, and also denied tobacco, alcohol, or drug use. He had a past medical history for hypertension but was not currently taking any medications. He denied any family history of neurologic, cardiac, or kidney disorders. Although the reason is unclear, we believe he waited 4 weeks to present to the hospital due to complex social reasons. The patient did not seek outside care prior to admission to the hospital.
Upon presentation to our facility, the patient’s vital signs were within normal limits (temperature 36.8°C, heart rate 86, respiratory rate 17, blood pressure 119/68) and a physical exam was performed. Cardiovascular assessment showed regular rate and rhythm with a normal S1/S2 without murmurs, rub, or gallop. Mental status assessment demonstrated normal attention, language, memory, concentration, and knowledge. Bilateral upper-extremity strength was rated 3/5 and bilateral lower-extremity strength was 1/5. The patient had intact sensation to touch in the upper extremities and paresthesia of the fingertips and plantar surface of the foot. Cranial nerves I-XII were intact. The patient was areflexic in the bilateral upper and lower extremities. He had a normal finger-to-nose test but was unable to perform a heel-to-shin test due to weakness. He tested negative for COVID-19.
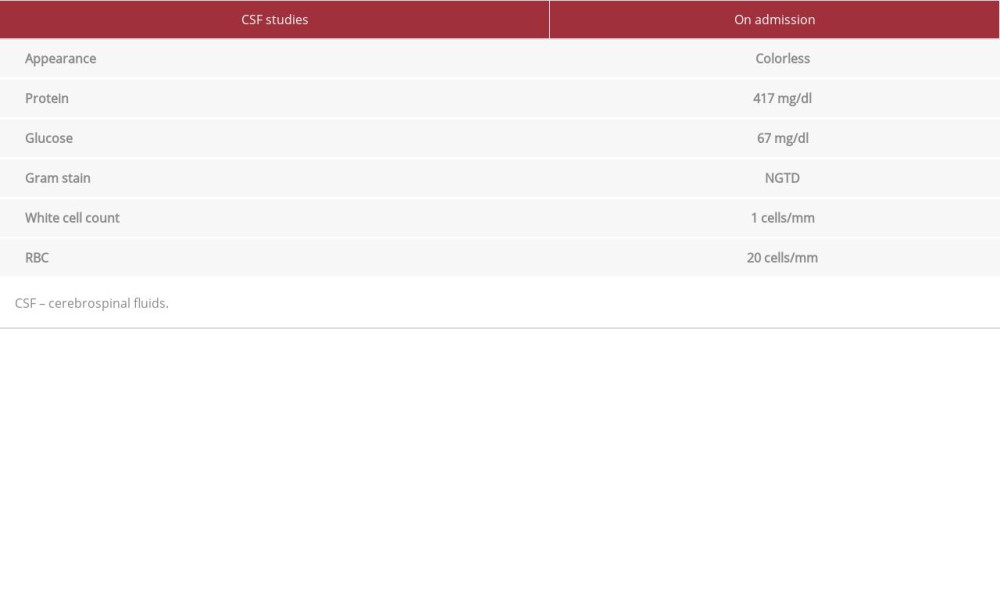
Regarding laboratory findings, the patient had a non-reactive treponemal, folate of 14.27 ng/ml, and elevated B12 of 1994 pg/ml. A lumbar puncture was performed and demonstrated an elevated protein level of 417 mg/dl and normal white blood cell count of 1 cell/mm3, consistent with albuminocyto-logic dissociation (Table 1). A computed tomography (CT) scan of the brain was unremarkable and magnetic resonance imaging (MRI) of the brain without contrast showed no acute diffusion or abnormal enhancement. The patient had an MRI of the lumbar, thoracic, and cervical spine as well, which showed degenerative changes with some stenosis, most significant at C5/6. The patient was categorized as Brighton Criteria level 2 based on absent deep-tendon reflexes, 72-h onset, bilateral weakness of limbs, cerebrospinal fluid (CSF) white blood cell count < 50, and CSF protein count greater than accepted normal values [8]. Our hospital does not have an inpatient nerve conduction study available, therefore one was not performed.
Based on the patient’s clinical symptoms and CSF findings, a diagnosis of GBS was made. A 5-day treatment with intravenous immunoglobulin was initiated. The patient failed to show improvement after the 5-day course and was therefore given a 5-day treatment of plasma exchange. He did not show improvement after the 5-day treatment with plasma exchange. He received physical therapy and inpatient rehabilitation with moderate improvement in his symptoms. He was discharged after 309 days of hospitalization without any notable events and without the need for ICU level of care. He was able to ambulate using a walker on discharge.
Discussion
Our case report demonstrates occurrence of GBS soon after the patient received the COVID-19 Moderna vaccine. Although he presented to our hospital 4 weeks after initial symptom onset, the clinical presentation of symmetric weakness and areflexia with an elevated cerebrospinal fluid protein level and normal white blood cell count supports the diagnosis of GBS. Our initial differential diagnoses included B12 deficiency and polyradiculopathy due to HIV. Laboratory test results reported a non-reactive HIV antigen antibody combination assay and a B12 level of 1994 pg/ml (normal limit 208–964 pg/ml).
There is evidence demonstrating an association of SARS-CoV-2 infection with an increased likelihood of GBS, with a prevalence of 0.015% in patients infected with SARS-CoV-2, although other epidemiological studies have refuted a causal relationship between SARS-CoV-2 and GBS [9,10]. Although there is an increased risk of GBS with COVID-19, there is still a weaker link between COVID-19 and GBS than with other more well-known pathogens classically associated with GBS [9]. The estimated overall incidence of GBS in the general population is 0.8–1.9/100 000 per year (9). Additionally, although there is no scientific evidence supporting a molecular mimicry mechanism, the association of GBS with COVID-19 may be better explained through an autoimmune-mediated mechanism causing an upregulation of inflammatory cytokines [9–12].
There is a known association of GBS following vaccine administration, most notably the influenza vaccine [13]. In a study examining GBS after influenza vaccination, 97.9% of patients developed symptoms within 3 weeks, with 54.2% of patients developing symptoms within the first 2 days of vaccine administration [14]. Our patient reported his symptoms began within 3 days after receiving his COVID-19 vaccine, which is consistent with the onset of GBS following other types of vaccination [14]. Additionally, our patient developed symptoms following his first dose of the COVID-19 vaccine, which is consistent with other case reports of GBS following COVID-19 vaccination [4].
Furthermore, there has been an increase in the number of reported cases of GBS following the Johnson-Johnson COVID-19 vaccine, which has led the Food and Drug Administration (FDA) to include a warning that the Johnson-Johnson COVID-19 vaccine might have an increased risk of GBS [15]. The FDA announced that of the 12.5 million doses of Johnson-Johnson vaccine administered, there were 100 preliminary reports of onset of GBS after vaccination, with 95 requiring hospitalization [15]. The FDA noted although there is an association with the Johnson-Johnson vaccine and an elevated risk of GBS, there is not adequate evidence to determine a causal relationship between GBS and the Johnson-Johnson vaccine [15]. It was also noted that there was no increase in GBS associated with the Moderna and Pfizer-BioNTech vaccines [15]. Additionally, the risk of developing GBS after COVID-19 vaccination is low and does not outweigh the benefits of receiving the COVID-19 vaccine [15]. Our patient’s symptoms following vaccine administration were reported to the Vaccine Adverse Effects Reporting System (VAERS).
Because our patient did not present to the hospital until 4 weeks after symptom onset, we were not able to test for other more common causes of GBS. Therefore, it is not possible to determine if our patient developed GBS due to the vaccine or if it was temporal in nature, resulting from a concomitant infection with a known GBS-causing pathogen. However, the patient did not report any infectious disease symptoms prior to vaccine administration. Unfortunately, our patient presented late in his disease course and will likely have permanent neurologic deficits.
Conclusions
This case should be taken as a cautionary tale for physicians to initiate prompt treatment if such symptoms arise after vaccination, even in the absence of imaging results, to prevent long-term disability.
References:
1.. Pilishvili T, Fleming-Dutra KE, Farrar JL, Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel – 33 U.S. sites, January-March 2021: MMWR Morb Mortal Wkly Rep, 2021; 70; 753-58
2.. : Selected Adverse Events Reported after COVID-19 Vaccination May 10, 2022, CDC [cited 2022 May 14]; Available from: URL: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3.. Koike H, Chiba A, Katsuno M, Emerging infection, vaccination, and Guillain-Barré syndrome: A review: Neurol Ther, 2021; 10; 523-37
4.. Finsterer J, Exacerbating Guillain-Barré syndrome eight days after vector-based COVID-19 vaccination: Case Rep Infect Dis, 2021; 2021; 3619131
5.. Hanson KE, Goddard K, Lewis N, Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the vaccine safety datalink: JAMA Netw Open, 2022; 5(4); e228879
6.. Yuki N, Hartung H-P, Guillain-Barré syndrome: N Engl J Med, 2012; 366; 2294-304
7.. Hughes RAC, Cornblath DR, Guillain-Barré syndrome: Lancet, 2005; 366(9497); 1653-66
8.. Ghazanfar H, Qazi R, Ghazanfar A, Iftekhar S, Significance of brighton criteria in the early diagnosis and management of Guillain-Barré syndrome: Cureus, 2020; 12(5); e8318
9.. Palaiodimou L, Stefanou MI, Katsanos AH, Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: A systematic review and meta-analysis: Eur J Neurol, 2021; 28(10); 3517-29
10.. Keddie S, Pakpoor J, Mousele C, Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome: Brain, 2021; 144(2); 682-93
11.. Dalakas MC, Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: Neurol Neuroimmunol Neuroinflamm, 2020; 7(5); e781
12.. Hussain FS, Eldeeb MA, Blackmore D, Guillain Barré syndrome and COVID-19: Possible role of the cytokine storm: Autoimmun Reviews, 2020; 19(12); 102681
13.. Willison HJ, Jacobs BC, van Doorn PA, Guillain-Barré syndrome: Lancet, 2016; 388(10045); 717-27
14.. Park Y-S, Lee K-J, Kim SW, Clinical features of post-vaccination Guillain-Barré syndrome (GBS) in Korea: J Kor Med Sci, 2016; 32(7); 1154-59
15.. : Coronavirus (COVID-19) Update: July 13, 2021, 2021, U.S. Food and Drug Administration. FDA [cited 2022 Jan]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-july-13-2021
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250