18 November 2022: Articles 
A 6-Day-Old Male Infant with Severe Hyperbilirubinemia Diagnosed with Hereditary Spherocytosis at a Tertiary Hospital in East Java, Indonesia: A Diagnostic and Management Challenge in a Developing Country
Challenging differential diagnosis, Unusual setting of medical care, Rare disease
Brigitta I.R.V. CorebimaDOI: 10.12659/AJCR.937416
Am J Case Rep 2022; 23:e937416
Abstract
BACKGROUND: Hereditary spherocytosis (HS) is an autosomal dominant inherited disorder that causes severe hyperbilirubinemia in neonates. There is no factual data about the prevalence in Indonesia. It is common that neonates with suspected hereditary spherocytosis are not diagnosed or treated adequately in developing countries such as Indonesia.
CASE REPORT: A 6-day-old baby was referred from a secondary public hospital to our tertiary hospital in Malang, East Java with severe hyperbilirubinemia unresponsive to the 2 days of conventional phototherapy. Initial laboratory examination showed total serum bilirubin level 28.83 mg/dL and indirect bilirubin level 25 mg/dL. Complete blood count showed hemoglobin level of 10.3 g/dL with high MCHC 36.9 g/dL and increased RDW 18.7%. The HS ratio (MCHC per MCV) was 0.41. The blood smear showed spherocytes with positive family history from the mother and grandmother. There were no specific tests such as EMA binding, cryohemolysis, or analysis of erythrocyte membrane protein available in our hospital. The patient was then treated with 2 sessions of intensive phototherapy with phototherapy unit bilisphere 360 LED. The total serum bilirubin level dropped to 12.19 mg/dL. In this case, we decided to perform intensive phototherapy first, not only because of facility-based constraints to do timely exchange transfusion, but also due to the low socio-economic and educational background of the parents.
CONCLUSIONS: There are some challenges in diagnosing and treating HS adequately in Indonesia. Limitations of specific tests, inadequacy of conventional phototherapy, lack of awareness of and adherence to guidelines, and facility-based inability to perform timely exchange transfusion all can contribute to severe hyperbilirubinemia and its sequelae.
Keywords: Hyperbilirubinemia, Neonatal, Phototherapy, Spherocytosis, Hereditary, Infant, Newborn, Male, Humans, Indonesia, Tertiary Care Centers, Developing Countries, Hyperbilirubinemia, bilirubin
Background
Hereditary spherocytosis (HS) is an inherited disorder in which the red blood cell structural proteins are abnormal, leading to the loss of membrane surface area and spherical-shaped, hyperdense, poorly deformable red blood cells. The pathogenesis of membrane surface area loss is due to mutation of genes encoding for membrane proteins such as ankyrin-1, band 3, β-spectrin, α-spectrin, and protein 4.2. The proportion of hereditary spherocytosis in severely jaundiced neonates is about 1%, about 30 times higher than that of overall population [1–4].
There is no actual data about the prevalence of hereditary spherocytosis in Asia, but it is estimated to be lower prevalence. Data from China in 36 years period found the prevalence was estimated to be 1.27 cases of 100 000 males and 1.49 cases of 100 000 females [5]. Inheritance of the disease is in an autosomal dominant fashion. However, 25% of all cases do not have a family history of spherocytosis. This may be caused by spontaneous mutations or recessive inheritance [6].
A neonate with HS usually shows severe hyperbilirubinemia and sometimes anemia in early life. A complete blood count usually shows a high mean corpuscular hemoglobin concentration (MCHC) but a low mean corpuscular volume (MCV). The blood smear usually shows spherocytes and polychromasia. More than half of the neonates with hereditary spherocytosis are not anemic in the first week of life, and splenomegaly is rarely found [3,7]. When hereditary spherocytosis is diagnosed, parents should be informed about the risks of recurrent anemia, jaundice, early gallstones, splenectomy, and its infection risks after splenectomy [8,9].
In Indonesia, there are many limitations regarding specific tests provided. Besides, lack of adherence to the guidelines, inadequacy of phototherapy devices in secondary hospitals, low socio-economic and education background of the parents, and facility-based constraints to do timely exchange transfusion all could make it more difficult to reduce severe hyper-bilirubinemia. This case report aims to present challenges in diagnosing and treating hereditary spherocytosis in neonates from a developing country, Indonesia.
Case Report
A-six-day old male baby was referred from a secondary public hospital to the emergency room of our tertiary hospital in Malang, Indonesia. The chief complaint of the baby was icterus since <24 hours after birth which was not improving despite 2 days of conventional phototherapy. The baby was also looked slightly pallor, sleepy, and difficult to awaken for feeding. Other complaints such as fever, vomiting, hypotonic/rigidity, high-pitched cry, or seizure were denied. Defecation and urination were expected; the baby defecated in <24 hours after birth with normal black color. The baby has been given conventional phototherapy for 2×24 hours in the previous hospital. After conventional phototherapy, the total bilirubin level in the previous hospital was still 32.3 mg/dL, the direct bilirubin level was 1.69 mg/dL, and the indirect one was 30.63 mg/dL.
The baby was the last child in the family. The older brother had history of recurrent pallor and packed red cell transfusion. The mother and grandmother had hereditary spherocytosis diagnosis from peripheral blood smear and bone marrow examination. From birth history, the baby was born by Caesarean section method in the 38–39 weeks of gestational age, indicating preeclampsia in the mother. There were neither history of maternal infection or delayed cord clamping from prenatal and perinatal history. Apgar scores were 6 and 7 in the first and fifth minutes, with neither respiratory distress nor bleeding or cephalhematoma seen in the baby. Vitamin K prophylaxis and hepatitis B immunization were administered to the baby. The baby’s birth weight was 2200 grams with 48 centimeters in length. Ponderal index was 1.9, indicating asymmetrical IUGR. Preeclampsia in the mother was the probable cause of asymmetrical IUGR in this baby. From history in the previous hospital, there were decrease of body weight during hospitalization for about 160 grams (7.2%). However, after 3 days of hospitalization in our hospital, the weight had been regained to 2150 grams. The baby previously was given low birth weight infant formula in addition to breastmilk.
From the general appearance, the baby looked moderately ill. The conjunctiva looked slightly anemic and icteric. From neck, chest, and abdominal examinations, there were no abnormalities.
When first admitted to our hospital, laboratory examination revealed that the total bilirubin level was 28.83 mg/dL (N: <1 mg/dL), the indirect level was 25 mg/dL (N: <0.75 mg/dL), and the direct level was 3.83 mg/dL (N: <0.25 mg/dL). Hemoglobin level decreases to 10.3 g/dL (N: 13.4–17.7 g/dL) with a hematocrit level of 27.9% (N: 40–47%). MCHC was slightly high with the level of 36.9 g/dL (N: 32–36 g/dL). The MCV level was 92.1 fL (N: 80–93 fL). Thus, the ratio of MCHC per MCV (HS ratio) was 0,4. RDW level was 18.7% (N: 11.5–14.5%), indicating anisocytosis. The blood smear of the baby showed anisopoikilocytosis, with spherocytes form accounting for about 21% of all erythrocytes (Figure 1). Other laboratory examinations such as liver and renal function tests, serum electrolytes, blood glucose, and marker of infection all were within normal limit as shown in Table 1. The baby’s blood group was the same as the mother A’s, with positive rhesus. Reticulocyte percentage increased to 6.81% (N: 0.5–2.5%). The Coombs’ test (Direct Antiglobulin Test) revealed a negative result.
The baby then got 2 sessions of phototherapy, 12 hours each, with intensive phototherapy unit bilisphere 360 LED. After intensive phototherapy, the total serum bilirubin level dropped to 12.19 mg/dL, the direct level dropped to 2.32 mg/dL, and the indirect level dropped to 9.87 mg/dL. The baby looked pallor with the hemoglobin evaluation on the second day of hospitalization showed the level of 9.1 g/dL; thus, we decided to give transfusion with 20 cc/kgs body weight of packed red cell. In the fourth day of hospitalization before being sent home, the hemoglobin evaluation revealed a normal value of 15.5 g/dL. We have informed the parents about the diagnosis and future complications that may occurred and arranged routine follow-up for this baby. However, the parents still refused to do the follow-up, because of the costs. The family was come from low socio-economic dan education background status. Besides, they did not join national health insurance programme.
Discussion
Based on the mechanism of bilirubin accumulation, there are 4 classifications of neonatal indirect hyperbilirubinemia cause. They are increased enterohepatic circulation, increased production, decreased clearance, and impaired conjugation. Increased enterohepatic circulation may be found in breast-milk jaundice or breastfeeding jaundice. Increased production may be caused by the hemolytic or non-hemolytic condition. Hemolytic condition characteristics are increased unconjugated bilirubin level, increased reticulocyte count (>6%), and hemoglobin level of <13 g/dL. Decrease clearance may be found in Gilbert syndrome, whereas impaired conjugation may be found in Crigler-Najjar syndrome. Other conditions which may cause indirect hyperbilirubinemia are maternal diabetes, pyloric stenosis, intestinal obstruction, congenital hypothyroidism, and bilirubin displacers [10,11].
The hemolytic condition could be accompanied by Coombs’ or Direct Antiglobulin Test (DAT) test positive (immune-mediated) or negative result. Examples of positive Coombs’ test conditions are ABO or rhesus incompatibility. In comparison, examples of negative Coombs’ test conditions are red blood cell membrane defect (spherocytosis), red blood cell enzyme defects (G6PD or pyruvate kinase deficiency), drug-cause such as streptomycin or vitamin K, abnormal red blood cells (hemoglobinopathies), and sepsis. In this case, there were increased unconjugated bilirubin level, increased reticulocyte count, anemia, and negative Coombs’ test. Thus, this patient’s possible cause of hyperbilirubinemia is a hemolytic condition with negative DAT test [2,10,12].
Indirect hyperbilirubinemia could also be classified as physiologic or pathologic ones. Physiologic jaundice is caused by normal bilirubin metabolism, usually characterized by the onset of jaundice after 24 hours of age, but resolves after 2–3 weeks of age in full-term infants. On the other hand, some features which may lead to suspicion of pathologic conditions are the onset of jaundice which is in the first 24 hours of age, rapid rise of total serum bilirubin level of more than 5 mg per dL per day, total serum bilirubin level is higher than the 95th percentile for age in hours based on age-specific bili-rubin normogram, and jaundice that persists beyond the age of 2–3 weeks. Some examples of underlying pathologic conditions are ABO disease, G6PD disorder, or hereditary spherocytosis. In this group of disorders, hyperbilirubinemia is sometimes severe and may lead to bilirubin-induced neurological dysfunction (BIND). There are some risk factors for severe neonatal hyperbilirubinemia and neurotoxicity, such as prematurity, autoimmune hemolytic disease, or glucose-6-phosphate dehydrogenase deficiency (G6PD deficiency), cephalohematoma or significant bruising, asphyxia, or sepsis [10,11].
We should exclude some differential diagnosis of indirect hyperbilirubinemia. In this patient, we did not find any signs of bruising, extravasated blood, dehydration, or sepsis. The baby had a history of weight fall to 2044 grams (decrease by 7%) in the previous hospital, but had regained his weight to the 2150 grams after 3 days of hospitalization in our hospital. Serum urea and creatinine, serum electrolyte, and blood glucose were normal. There were no signs of infection, such as fever of temperature instability. Marker of infection (procalcitonin) was normal, and blood culture was sterile.
There are 5 primary genes in the pathogenesis of hereditary spherocytosis, which are α-spectrin (SPTA1), β-spectrin (SPTB), ankyrin (ANK1), band 3 (SLC4A1), and protein 4.2 (EPB42). Mutations in ≥1 of HS-genes may cause membrane protein deficiency and thus lead to hemolysis (Figure 2) [13,14].
According to some studies, high MCHC and RDW in a neonate with severe hyperbilirubinemia may alert physicians to the possibility of hereditary spherocytosis. An MCHC of ≥36 g/dL had 82% sensitivity and 98% specificity for diagnosing hereditary spherocytosis [15]. The possibility of hereditary spherocytosis can also be measured by dividing MCHC with MCV, known as HS ratio. The value of >0.36 indicates that HS is present with 97% sensitivity, >99% specificity, and >99% negative predictive value [16]. HS ratio should be obtained from a complete blood count drawn in the first week after birth. The blood smear of HS patients should show spherocytes and polychromasia [17]. In this patient, HS ratio was 0.41. The blood smear also showed spherocytes (Figure 1). We should suspect that the patient had hereditary spherocytosis disorder from these data.
From the British Journal of Hematology guideline, a patient with a positive family history of HS, together with typical clinical features and laboratory examinations (spherocytes, raised MCHC, an increase of reticulocytes) is diagnosed with hereditary spherocytosis without a need of additional tests (grade I recommendation, grade A evidence). However, if the diagnosis is equivocal, then a screening test with a high predictive value of HS is needed. The recommended screening tests are the cryohemolysis test and EMA binding (grade I recommendation, grade A evidence). We concluded that the patient had a hereditary spherocytosis diagnosis because of positive family history, typical clinical features, and laboratory examinations [9]. In this case, there was no need to continue to specific tests such as the cryohemolysis test and EMA binding test. Also, these tests were not provided in our hospital.
Based on the Indonesia Basic Health Research 2007, data on the causes of neonatal death, hyperbilirubinemia is the number 5th cause of early neonatal (0–6 days) mortality with the prevalence of 5.6% after respiratory insult, prematurity, sepsis, and hypothermia. There is no national data in Indonesia regarding severe hyperbilirubinemia. The biggest data from 8 hospitals in 3 regions of Indonesia showed that the prevalence of severe hyperbilirubinemia was 6,8% with acute bili-rubin encephalopathy was 2,2%. The data about kernicterus itself is lacking in Indonesia till now [18].
The patient in this case is an example of delayed referral case. As we know, in low-middle income countries, there are many limitations regarding diagnosing and treating severe hyper-bilirubinemia cases. Limitations include delay in diagnosing and treating neonates with severe hyperbilirubinemia. The patient had a history of 2 days of conventional phototherapy in secondary hospital despite ineffective results. This might be caused by lack of awareness and adherence of healthcare workers to hyperbilirubinemia guidelines and the inadequacy of phototherapy devices in some hospitals in Indonesia [19,20].
Data from a large research to Indonesian healthcare workers, it was concluded that almost 30% of the midwives and 23% of general practitioners were not aware of hyperbilirubinemia guidelines by American Academy of Pediatrics/WHO/ Indonesian Pediatric Society, or they did not adhere to them. Furthermore, only 54% of the midwives, 68% of the general practitioners, and 89% of the pediatricians know the warning signs of severe hyperbilirubinemia correctly. Twenty-eight percent of the midwives and 31% of the general practitioners planned the first follow-up visit after 72 hours with 90% of them discharged the infants less than 48 hours after birth. It concluded that the awareness of and adherence to the guidelines is still lacking amongst midwives and general practitioners in Indonesia [19]. Another challenge is inadequacy of photo-therapy in some hospitals in Indonesia. Data from 17 hospitals with level II and III Neonatal Intensive Care Units (NICUs) facility in Java, Indonesia, from 77 combinations of 20 different phototherapy devices, it showed that in 9 hospitals the irradiance levels were less than 10 µW/cm2/nm, level required for conventional phototherapy [20].
When first admitted to our tertiary hospital, the patient’s total serum bilirubin (TSB) level was still 28.83 mg/dL at 6 days old. According to the phototherapy guidelines from the American Academy of Pediatrics for hospitalized infants ≥35 weeks, the total serum bilirubin level was above the indicated level of exchange transfusion, which was above 22.5 mg/dL for the neonate with medium risk in the age of 144 hours [21]. In this patient B/A ratio (bilirubin per albumin ratio) was also high 7.7. Infants ≥38 0/7 weeks of gestation with clinically higher risk/isoimmune hemolytic disease/G6PD deficiency should get exchange transfusion if the ratio is ≥7.2. However, we decided to give intensive phototherapy first, because of some limitations to undergo timely exchange transfusion. Total serum bilirubin and B/A ratio both are both strong predictors for neurotoxicity [22].
In this case, there are some causes which postpone the exchange transfusion procedure. First, the father refused to undergo the procedure, because of low education dan socio-economic background. Besides, there were other limitations which may delay the procedure in our hospital, such as get the blood samples from the mother for cross-match evaluation (the mother did not come along), transportation of blood samples to and collection of cross-matched blood from the laboratory, preparing staff and equipment for exchange transfusion. These facility-based constraints all may delay timely exchange transfusion process. These are common problems which usually found in low-middle income countries [23]. This could expose the baby to a higher risk of acute bilirubin encephalopathy or kernicterus, thus we decide to give intensive phototherapy first.
Bilirubin level should be decreased urgently, because extreme hyperbilirubinemia with total serum bilirubin >25 mg/dL may lead to death, acute bilirubin encephalopathy (ABE) and/or bilirubin-induced neurologic dysfunction (BIND), also subsequent chronic bilirubin encephalopathy (kernicterus) [24]. Modified bilirubin-induced neurologic dysfunction (BIND-M) scoring system is used in low-resource testing where specific tests such as MRI and ABRs (Auditory Brain Stem Responses) are not feasible. Total score of ≥3 is highly predictive of clinical ABE with sensitivity of 90.7%, specificity of 97.7%, positive predictive value of 88,9%, and negative predictive value of 98.2% [25]. In this patient, the total score was 1 with clinically sleepy and difficult to awaken for feeding.
The patient in this case is classified as ‘moderate’ form of hereditary spherocytosis. Children in moderate or severe group might need to undergo splenectomy, especially if complication such as gallstone formation found. Besides, children in moderate or severe group should also receive folate supplementation to prevent folic acid deficiency [9]. We have educated the parents about the risks and arranged follow-up plans, but they rejected to undergo the plans because could not afford it. We gave the patient packed red cell transfusion and 50 mcg of folic acid supplementation before sent him home. From the last laboratory examination evaluation before being sent home, hemoglobin increased to 15.5 g/dL with a total serum bilirubin level of 12.19 mg/dL.
Because of the challenges in diagnosing and treating hyper-bilirubinemia in Indonesia, in 2019, the Indonesian Pediatric Society launched a national guideline for diagnosing and treating infants with hyperbilirubinemia. In this guidelines, it is also explained a new web-based application named ‘Bilinorm’ to help clinicians decide whether the babies need phototherapy or exchange transfusion [26]. When this application first introduced, healthcare workers and pediatric residents in our teaching hospital in Malang, East Java, were also trained to use this application. After 6 months the introduction of the app, we filled questionnaires about hyperbilirubinemia management in our hospital after using this app. Statistical analysis of that research including 2 teaching hospitals in East Java showed that there were significantly more infants received treatment according to the guidelines (38% vs 51%,
In our teaching hospital in Malang, besides using AAP normo-gram, ‘Bilinorm’ application, and national guideline we also have tried to improve hyperbilirubinemia treatment practice in our hospital by routinely conduct training for pediatric residents and healthcare workers in neonatology ward and neonatal intensive care units. Training included how to diagnose hyperbilirubinemia, how to use those guidelines to treat hyperbilirubinemia, what the signs of severe hyperbilirubinemia, and how to do exchange transfusion procedure. We have done few times of exchange transfusion procedures in our NICU, but not all infants who required the procedure could get the procedure because of some limitations in our country as stated above. This training might also be needed in the future for healthcare workers in primary or secondary settings. Socio-economic, education, religious background of the parents in Indonesia sometimes make the effective treatment more difficult to do.
Conclusions
In the developing country such as in our country, there are some challenges in diagnosing and treating infants with severe hyperbilirubinemia. Limitation of specific tests, awareness or adherence of general practitioners or midwives to guidelines, inadequacy of phototherapy devices in secondary or primary settings, and various socio-economic, education, and also religious background of parents in Indonesia all could make a delay in the hyperbilirubinemia diagnosis and treatment. Training and socialization of hyperbilirubinemia guidelines could be some options to improve severe hyperbilirubinemia treatment.
Figures
References:
1.. Iolascon A, Avvisati RA, Piscopo C, Hereditary spherocytosis: Transfus Clin Biol, 2010; 17(3); 138-42
2.. Mahajan V, Jain SK, Hereditary spherocytosis: NeoReviews, 2016; 17(12); e697-e704
3.. Christensen RD, Yaish HM, Gallagher PG, A Pediatrician’s practical guide to diagnosing and treating hereditary spherocytosis in neonates: Pediatrics, 2015; 135(6); 1107-14
4.. Saada V, Cynober T, Brossard Y, Incidence of hereditary spherocytosis in a population of jaundiced neonates: Pediatr Hematol Oncol, 2006; 23(5); 387-97
5.. Wang C, Cui Y, Li Y, A systematic review of hereditary spherocytosis reported in Chinese biomedical journals from 1978 to 2013 and estimation of the prevalence of the disease using a disease model: Intractable Rare Dis Res, 2015; 4(2); 76-81
6.. Shah S, Vega R, Hereditary spherocytosis: Pediatr Rev, 2004; 25(5); 168-72
7.. Delhommeau F, Cynober T, Schischmanoff PO, Natural history of hereditary spherocytosis during the first year of life: Blood, 2000; 95(2); 393-97
8.. Perrotta S, Gallagher PG, Mohandas N, Hereditary spherocytosis: Lancet, 2008; 372(9647); 1411-26
9.. Bolton-Maggs PHB, Langer JC, Iolascon A, Guidelines for the diagnosis and management of hereditary spherocytosis – 2011 update: Guideline: Br J Haematol, 2012; 156(1); 37-49
10.. Porter M, Dennis B, Hyperbilirubinemia in the term newborn: Am Fam Physician, 2002; 65(4); 599-607
11.. Anderson NB, Calkins KL, Neonatal indirect hyperbilirubinemia: Neoreviews, 2020; 21(11); e749-60
12.. Ullah S, Rahman K, Hedayati M, Hyperbilirubinemia in neonates: Types, causes, clinical examinations, preventive measures and treatments: A narrative review article: Iran J Public Health, 2016; 45(5); 558-68
13.. He BJ, Liao L, Deng ZF, Molecular genetic mechanisms of hereditary spherocytosis: Current perspectives: Acta Haematol, 2018; 139(1); 60-66
14.. Aster J, Red blood cell and bleeding disorders: Pathologic Basis of Disease, 2005; 626, Philadelphia, PA, Elsevier Saunders
15.. Christensen RD, Henry E, Hereditary spherocytosis in neonates with hyper-bilirubinemia: Pediatrics, 2010; 125(1); 120-25
16.. Yaish HM, Christensen RD, Henry E, A simple method of screening newborn infants for hereditary spherocytosis: J Applied Hematol, 2013; 4(1); 27-32
17.. Christensen RD, Yaish HM, Lemons RS, Neonatal hemolytic jaundice: Morphologic features of erythrocytes that will help you diagnose the underlying condition: Neonatology, 2014; 105(4); 243-49
18.. Greco C, Arnolda G, Boo NY, Neonatal jaundice in low- and middle-income countries: Lessons and future directions from the 2015 Don Ostrow Trieste Yellow Retreat: Neonatology, 2016; 110(3); 172-80
19.. Sampurna M, Ratnasari K, Etika R, Adherence to hyperbilirubinemia guidelines by midwives, general practitioners, and pediatricians in Indonesia: PLoS One, 2018; 13(4); e0196076
20.. Sampurna MTA, Ratnasari KA, Saharso D, Current phototherapy practice on Java, Indonesia: BMC Pediatrics, 2019; 19(188); 188
21.. , Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation [published correction appears in Pediatrics. 2004;114(4): 1138]: Pediatrics, 2004; 114(1); 297-316
22.. Iskander I, Gamaleldin R, El Houchi S, Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy: Pediatrics, 2014; 134(5); e1330-39
23.. Mabogunje CA, Olaifa SM, Olusanya BO, Facility-based constraints to exchange transfusions for neonatal hyperbilirubinemia in resource-limited settings: World J Clin Pediatr, 2016; 5(2); 182-90
24.. Usman F, Diala U, Shapiro S, Acute bilirubin encephalopathy and its progression to kernicterus: Current perspectives: Res Rep Neonatol, 2018; 8; 33-44
25.. Radmacher PG, Groves FD, Owa JA, A modified Bilirubin-induced neurologic dysfunction (BIND-M) algorithm is useful in evaluating severity of jaundice in a resource-limited setting: BMC Pediatr, 2015; 15; 28
26.. : [National Guidelines for Medical Services for the Management of Hyperbilirubinemia.], 2019, Jakarta, Menteri Kesehatan Republik Indonesia (Indonesian). Available at: https://www.idai.or.id/professional-resources/pedoman-konsensus/pedoman-nasional-pelayanan-kedokteran-tata-laksana-hiperbilirubinemia
27.. Sampurna MTA, Ratnasari KA, Irawan ZS, Evaluation of a mobile application tool (BiliNorm) to improve care for newborns with hyperbilirubinemia in Indonesia: PLoS One, 2022; 17(6); e0269286
Figures
Tables
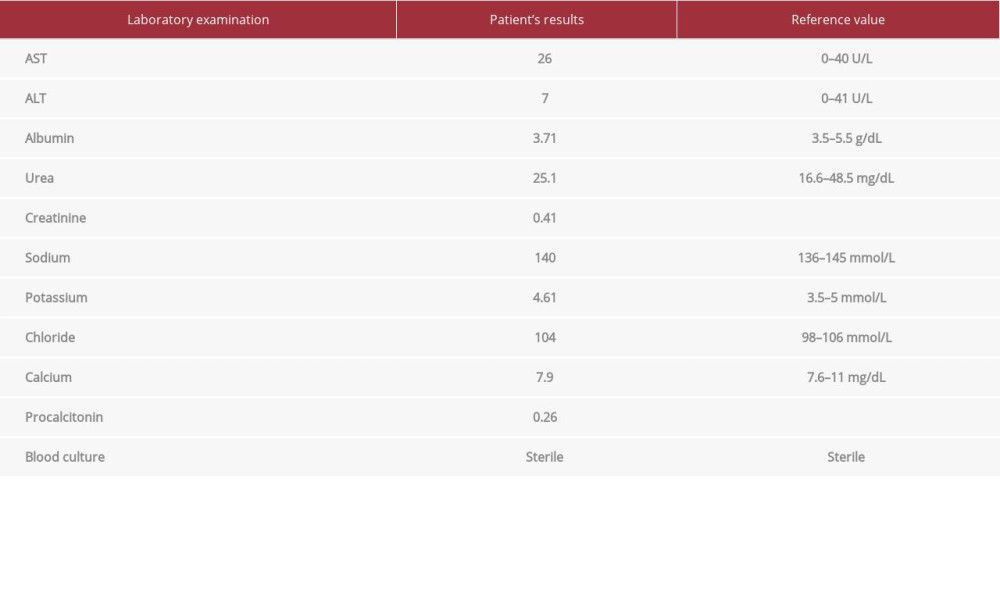 Table 1.. Patient’s laboratory examination results.The patient’s laboratory examination results show normal results of liver and renal function tests, serum electrolytes, and also marker of infection.
Table 1.. Patient’s laboratory examination results.The patient’s laboratory examination results show normal results of liver and renal function tests, serum electrolytes, and also marker of infection. Table 1.. Patient’s laboratory examination results.The patient’s laboratory examination results show normal results of liver and renal function tests, serum electrolytes, and also marker of infection.
Table 1.. Patient’s laboratory examination results.The patient’s laboratory examination results show normal results of liver and renal function tests, serum electrolytes, and also marker of infection. In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








