21 February 2023: Articles 
Pharmacogenetic Testing in a 70-Year-Old Woman with Polypharmacy and Multiple Comorbidities: A Case Report
Unknown etiology, Adverse events of drug therapy
Jayson P. Jessop1ABCDEF, Joshua Russell1ABCDEF, Adriana DeJesus1ABEF, Chandni Bardolia1ADEF, Abeer Hanna2BCD, Jacques Turgeon34ADEF*, Veronique Michaud43ABDEF, Nishita S. Amin1ABCDEFDOI: 10.12659/AJCR.938850
Am J Case Rep 2023; 24:e938850
Abstract
BACKGROUND: Comorbidities and polypharmacy are difficult to manage, as polypharmacy hinders identification and prevention of medication-related problems. Risk for adverse drug events (ADEs) can be minimized through pharmacogenomic (PGx) testing and related therapeutic adjustments.
CASE REPORT: A 70-year-old woman with comorbidities and medications enrolled in the Program of All-inclusive Care for the Elderly presented with left lower extremity (LLE) pain, generalized weakness, and major depressive disorder. The provider requested a medication safety review, where the clinical pharmacist-recommended PGx testing given the LLE pain and weakness while taking a statin and inconsistent INR readings taking warfarin. The pharmacist recommended switching atorvastatin to pravastatin to minimize the risk for statin-associated ADEs due to CYP3A4 inhibition and switching fluoxetine to citalopram due to uncontrolled depression/anxiety and to mitigate drug-drug interactions with carvedilol to reduce the risk of orthostatic hypotension. Recommendations were accepted and upon follow-up the patient reported minor LLE pain and improved wellbeing on citalopram. Following PGx testing, the patient had decreased function at SLCO1B1 and was an intermediate metabolizer for CYP2C9 and CYP2D6. This case demonstrates how preemptive PGx testing would have identified drug-gene interactions (DGIs) at the time of prescribing and reduced the risk of statin-associated muscular symptoms, highlighting the utility of panel-based PGx testing in older adults at high risk for ADEs and/or therapy failure.
CONCLUSIONS: Decreased function at SLCO1B1 increases exposure to statins, leading to statin-induced myalgias, as displayed in this case. PGx testing can help identify DGIs, choose optimal therapies in medically complex older adults, and minimize ADE risk.
Keywords: Drug-Related Side Effects and Adverse Reactions, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Myalgia, Pharmacogenetics, Aged, Female, Humans, citalopram, Depressive Disorder, Major, Liver-Specific Organic Anion Transporter 1, Pain, Pharmacogenomic Testing, Polypharmacy
Background
As national demographics shift towards an older population, comorbidities and polypharmacy are becoming more difficult to manage across healthcare disciplines [1,2]. Approximately 23% of Medicare patients have 5 or more comorbidities [3], and 39% of adults 65 years or older take 5 or more medications per day [4]. Polypharmacy complicates the identification and prevention of medication-related problems (MRPs), as drug-drug interactions (DDIs) increase with the total number of medications in a regimen [5].
In addition to polypharmacy-related risks, older adults are more vulnerable to adverse drug events (ADEs) due to physiological changes that occur with aging (eg, decreased blood pressure, pulse, respiratory rate, and loss of body fat and muscle to regulate temperature) [6]. These risks are higher in adults taking statins and warfarin, with as many as 10% of patients experiencing myalgia with statins and up to 7% experiencing a clinically significant bleed on warfarin [7,8].
The probability of experiencing ADEs can be minimized through appropriate testing, like pharmacogenomic (PGx) testing and related therapeutic adjustments [9,10]. Pharmacists trained in PGx are well-positioned as members of interdisciplinary health-care teams to interpret PGx results, recommend actions to mitigate MRPs, and educate clinicians, especially to help guide statin and warfarin therapy [9]. This case report demonstrates how preemptive PGx testing, while utilizing a clinical decision support system (CDSS), would have identified drug-gene and drug-drug interactions at the time of prescribing, resulting in reduced risk of ADEs [11]. The aim of this case report was to perform a pharmacogenetic examination in a 70-year woman with polypharmacy and multiple comorbidities.
Case Report
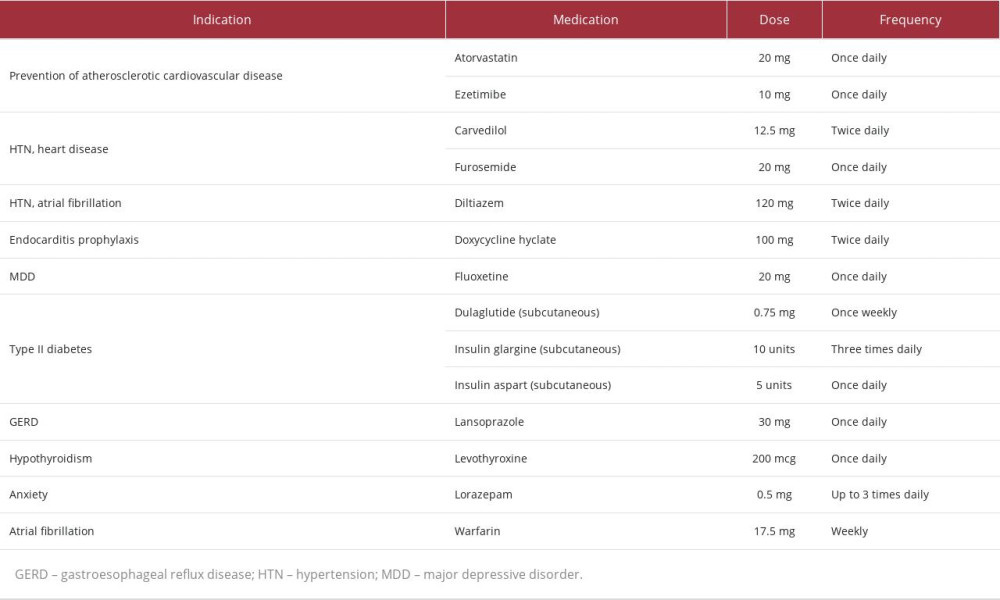
A 70-year-old woman with multiple comorbidities and medications (Table 1) enrolled in the Program of All-inclusive Care for the Elderly (PACE) on December 1, 2021 (Day 1). Upon enrollment, the patient presented with left lower extremity (LLE) pain, generalized weakness, and major depressive disorder (MDD). A Patient Health Questionnaire-9 (PHQ-9) evaluation suggested mild depression. At enrollment, the patient’s high-density lipoprotein (HDL), low-density lipoprotein (LDL), and total cholesterol (TC) were 47, 97, and 176mg/dL, respectively. Considering this information, the patient’s atherosclerotic cardiovascular disease (ASCVD) risk of 20.3% indicated high-intensity statin therapy despite her reported LLE pain and high risk of statin intolerance due to specific risk factors [1].
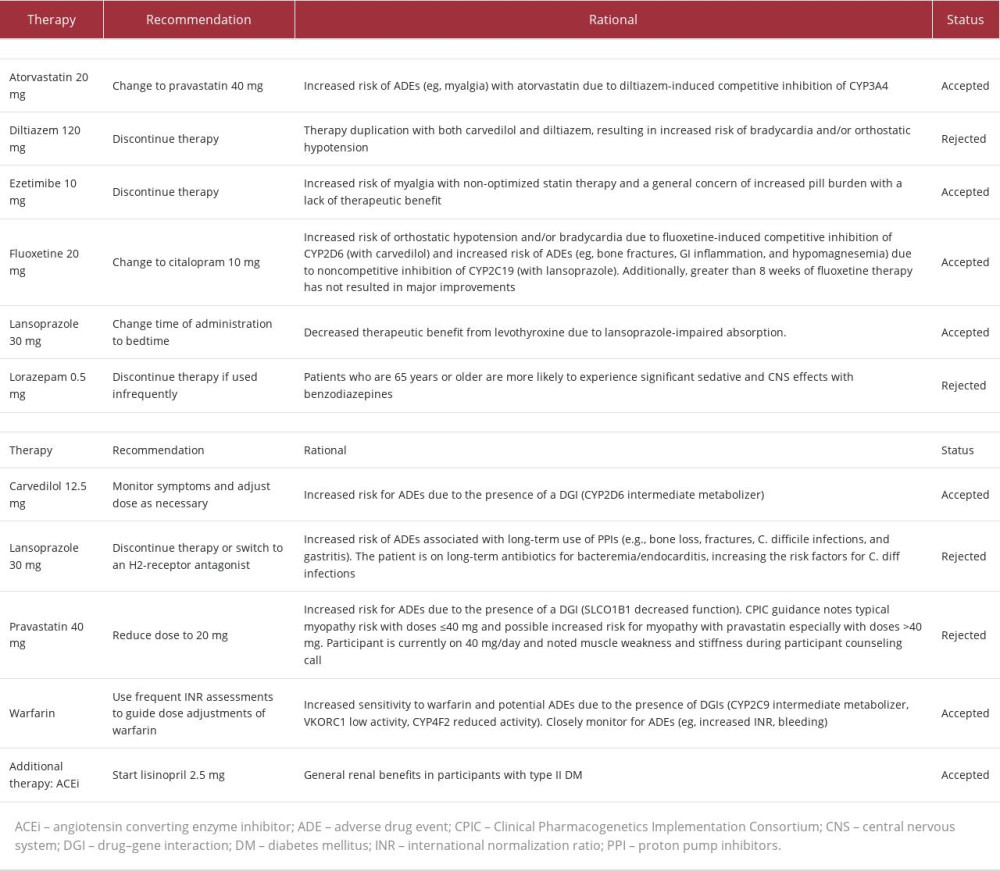
The patient’s care team requested that a medication safety review be performed by a clinical pharmacist. Shortly after enrollment, the clinical pharmacist made 11 recommendations (listed in Table 2) to improve the safety and efficacy of the patient’s medication regimen; 3 changes were implemented on Day 36. First, fluoxetine was changed to citalopram due to 1) mild depression after greater than 8 weeks of therapy, 2) the potential for fluoxetine to cause mechanism-based inhibition of lansoprazole metabolism via cytochrome P450 (CYP) 2C19 (CYP2C19), and 3) competitive inhibition of carvedilol metabolism via CYP2D6. Secondly, ezetimibe was discontinued to reduce pill burden. Then, atorvastatin 20 mg was changed to pravastatin 40 mg to minimize potential interactions with diltiazem and warfarin at CYP3A4 and to reduce the risk of continued LLE pain and generalized weakness. An updated medication list following the implementation of these pharmacist-recommended medication changes can be found in Table 2.
The patient’s “time in therapeutic range” for warfarin dosing (ie, an international normalization ratio (INR) goal between 2.5 and 3.5) was less than 50%, a consistent trend throughout the patient’s history with warfarin. Unstable INR readings were observed despite a self-reported adherence to warfarin and consistent diet. At the recommendation of a clinical pharmacist, the clinician ordered a PGx test on Day 87 to optimize the patient’s complex medication regimen.
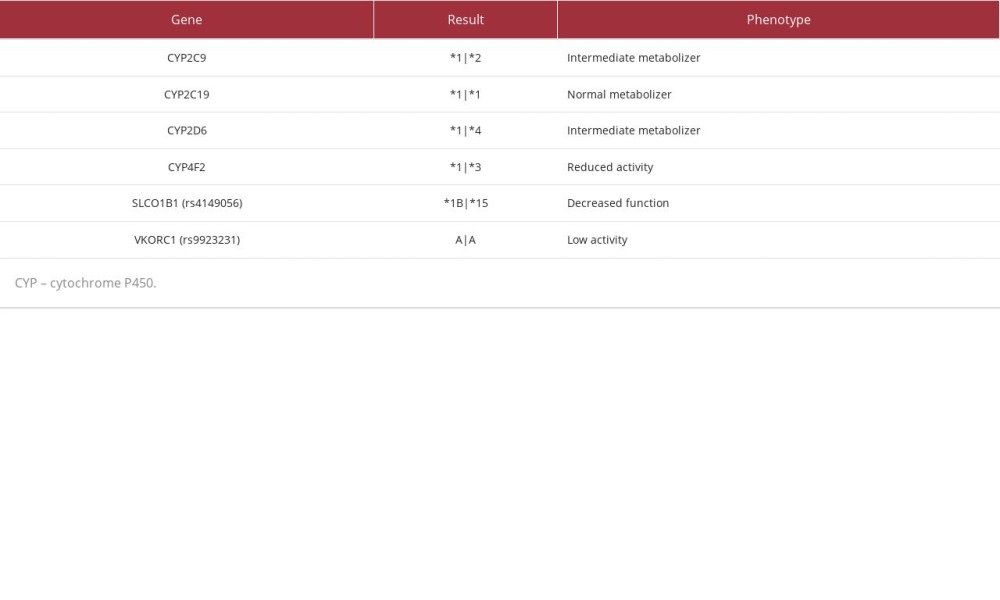
PGx results (Table 3) revealed decreased function of solute carrier organic anion transporter family member 1B1 (SLCO1B1), a transporter protein that facilitates statin entry into hepatocytes. Utilizing these results and a CDSS (MedWise®), the PGx pharmacist recommended close monitoring for signs of myopathy, and decreasing pravastatin to 20 mg, if deemed clinically appropriate based on an updated lipid panel.
The cumulative effect of the patient’s VKORC1, CYP4F2, and CYP2C9 status was an overall increased warfarin sensitivity [12,13]. As warfarin has a stronger affinity for the CYP2C19 enzyme than citalopram, the pharmacist noted a drug-drug-gene interaction (DDGI) between warfarin and citalopram – warfarin-mediated competitive inhibition of co-administered citalopram increases risk of ADEs. The pharmacist recommended minimizing this risk by separating the time of administration.
Other medication-related recommendations made over the course of care can be found in Table 2.
During follow-up assessments, the patient has reported minor LLE pain primarily occurring at night. Although a second PHQ-9 evaluation was not conducted, patient wellbeing had improved. Eight months after enrollment, HDL, LDL, and total cholesterol were 51, 70, and 134 mg/dL, respectively – a 28% LDL reduction and 24% TC reduction.
Although the patient still experiences wide INR ranges, the team closely monitors INR readings and makes appropriate dose adjustments. Diet education has also been provided to the patient to help minimize future INR fluctuations. Eight months after enrollment, the patient’s INR is checked every 3 to 4 days. The patient’s provider reports her INR is more stable following the pharmacist’s education on strategies to avoid large INR fluctuations. Within the CDSS, the patient’s MedWise® Risk Score™ decreased from high risk to moderate risk. The patient reported improved mental and physical health since enrollment in PACE and implementation of pharmacist-recommended interventions.
Discussion
This case report offers an example of the full utility of panel-based PGx testing in older adults who are at high risk of ADEs and/or therapy failure. PGx results exposed additional risk factors for statin-induced myopathy that have been linked to increased serum statin concentrations and greater likelihood of experiencing myopathy and/or generalized weakness [14].
The patient reported LLE pain, which was attributed to trauma from a motor vehicle accident that occurred years prior to enrollment. Statin therapy is known to reduce the threshold of experiencing muscle pain. Statin-associated muscular symptoms (SAMS) risk factors include female gender, age greater than 65 years, hypothyroidism, diabetes, and trauma; this patient presented with all 5 risk factors. Obtaining PGx results prior to prescribing the statin, also known as preemptive PGx testing, would have enabled the healthcare team to identify and minimize the risk of SAMS [14,15].
In 2022, the Clinical Pharmacogenetics Implementation Consortium (CPIC) updated its statin-SLCO1B1 guideline to emphasize the SAMS risk evidence based on statin therapy intensity [12]. In this case, the patient was taking atorvastatin 20 mg at time of enrollment. Correlation between dose and SAMS risk can be seen with every statin; however, the highest SAMS risk is associated with atorvastatin, simvastatin, pitavastatin, and lovastatin [12]. At enrollment, it was determined that maintaining moderate-intensity statin therapy was appropriate for our patient based on her ASCVD risk [16]. Per the CPIC guidelines, pravastatin 40 mg was more appropriate than atorvastatin because of its lower risk of SAMS. Although atorvastatin 20 mg is also an appropriate option due to its low SAMS risk, her co-prescribed diltiazem and warfarin both have a stronger affinity for the CYP3A4 enzyme than atorvastatin, resulting in DDIs, higher serum levels of atorvastatin, and, ultimately, elevated atorvastatin-associated SAMS risk [12,17]. For these reasons, atorvastatin may have contributed to the development of LLE pain.
Unlike atorvastatin, pravastatin is not extensively metabolized by the CYP system, making pharmacokinetic DDIs significantly less likely, especially in this case and others with SCLO1B1 decreased function. Evidence suggests that SLCO1B1 decreased function may also decrease the risk-modifying benefits of statin therapy [18,19]. Mechanistically, this may be explained by reduced SLCO1B1-mediated importation of statins into hepatic cells, where HMG-CoA reductase, the primary enzymatic target of statins, plays a role in cholesterol biosynthesis. The benefits of statin dose changes should always be weighed against these increased risks.
A wide range of therapy options exists for patients who do not tolerate statin therapy. These alternatives should be considered on an individual basis due to variable benefit and side effect profiles. Statin alternatives include PCSK9 inhibitors, bempedoic acid, fibric acid derivatives, peroxisome proliferator-activated receptor alpha (PPAR-alpha) agonists, niacin, bile acid sequestrants, and ezetimibe [20]. These alternatives should be considered if statin therapy alone is no longer sufficient to manage a patient’s hyperlipidemia, despite being optimized [16]. The recommendation to remove ezetimibe was made prior to PGx testing. However, if PGx test results were available at the time of enrollment, this recommendation would have likely not been made.
Current evidence supports the use of PGx-guided dosing for warfarin initiation. However, in this case, the patient had been taking warfarin for more than a decade prior to enrollment. Given the diagnoses of atrial fibrillation and the presence of a mechanical mitral valve, warfarin was deemed the most appropriate anticoagulant for this patient [16]. When PGx results are available at the time of warfarin initiation, polymorphisms identified in the VKORC1, CYP4F2, and CYP2C9 genes can guide calculation of initiation doses [21]. Our patient’s polymorphisms in the VKORC1 and CYP2C9 genes could result in increased warfarin sensitivity, while her polymorphism in CYP4F2 could result in decreased warfarin sensitivity. Collectively, these mutations make increased sensitivity a more probable outcome. The utility of this data is highest early in therapy; clinicians should consider ordering tests as early as available and, if possible, prior to initiation [21].
Conclusions
Decreased function of the SLCO1B1 transporter can increase exposure to statins, leading to statin-induced myalgias, as displayed in this case. PGx can help clinicians choose optimal therapies for medically complex older adults, minimizing the risk of ADEs. Specifically, preemptive panel-based PGx testing supports reduction of the risk of statin-induced myalgia/myopathies. When starting statin therapy or in the event of SAMs, PGx testing is especially beneficial to help prevent negative outcomes while improving quality of life.
References:
1.. Virani SS, Morris PB, Agarwala A, ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: A report of the American College of Cardiology Solution Set Oversight Committee: J Am Coll Cardiol, 2021; 78(9); 960-93
2.. Lothian K, Philp I, Maintaining the dignity and autonomy of older people in the healthcare setting: BMJ, 2001; 322(7287); 668-70
3.. Davis JW, Chung R, Juarez DT, Prevalence of comorbid conditions with aging among patients with diabetes and cardiovascular disease: Hawaii Med J, 2011; 70(10); 209-13
4.. Charlesworth CJ, Smit E, Lee DS, Polypharmacy among adults aged 65 years and older in the United States: 1988–2010: J Gerontol A Biol Sci Med Sci, 2015; 70(8); 989-95
5.. Khandeparkar A, Rataboli PV, A study of harmful drug-drug interactions due to polypharmacy in hospitalized patients in Goa Medical College: Perspect Clin Res, 2017; 8(4); 180-86
6.. Chester JG, Rudolph JL, Vital signs in older patients: Age-related changes: J Am Med Dir Assoc, 2011; 12(5); 337-43
7.. Ramkumar S, Raghunath A, Raghunath S, Statin therapy: Review of safety and potential side effects: Acta Cardiol Sin, 2016; 32(6); 631-39
8.. Gomes T, Mamdani MM, Holbrook AM, Rates of hemorrhage during warfarin therapy for atrial fibrillation: CMAJ, 2013; 185(2); E121-27
9.. Jarvis JP, Peter AP, Keogh M, real-world impact of a pharmacogenomics-enriched comprehensive medication management program: J Pers Med, 2022; 12(3); 421
10.. Meaddough EL, Sarasua SM, Fasolino TK, Farrell CL, The impact of pharmacogenetic testing in patients exposed to polypharmacy: A scoping review: Pharmacogenomics J, 2021; 21(4); 409-22
11.. Elchynski AL, Cicali EJ, Ferrer Del Busto MC, Determining the potential clinical value of panel-based pharmacogenetic testing in patients with chronic pain or gastroesophageal reflux disease: Pharmacogenomics J, 2021; 21(6); 657-63
12.. , Clinical Pharmacogenetics Implementation Consortium Guidelines [online] 2020 August 11. [cited 26 April 2022]. Available from: URL: https://cpicpgx.org/guidelines
13.. , Pharmacogenetics [online] [cited 24 April 2022] Available from: URL: https://www.knmp.nl/downloads/pharmacogenetic-recommendations
14.. Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M, Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin: Clin Pharmacol Ther, 2007; 82(6); 726-33
15.. Feng Q, Wilke RA, Baye TM, Individualized risk for statin-induced myopathy: Current knowledge, emerging challenges and potential solutions: Pharmacogenomics, 2012; 13(5); 579-94
16.. January CT, Wann LS, Calkins H, AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons: Circulation, 2019; 140; e125
17.. Zhou S, Chan E, Li X, Huang M, Clinical outcomes and management of mechanism-based inhibition of cytochrome P450 3A4: Ther Clin Risk Manag, 2005; 1(1); 3-13
18.. Wu X, Gong C, Weinstock J, Associations of the SLCO1B1 polymorphisms with hepatic function, baseline lipid levels, and lipid-lowering response to simvastatin in patients with hyperlipidemia: Clin Appl Thromb Hemost, 2018; 24(9 Suppl.); 240S-47S
19.. Oni-Orisan A, Hoffmann TJ, Ranatunga D, Characterization of statin low-density lipoprotein cholesterol dose-response using electronic health records in a large population-based cohort: Circ Genom Precis Med, 2018; 11(9); e002043
20.. Bardolia C, Amin NS, Turgeon J, Emerging non-statin treatment options for lowering low-density lipoprotein cholesterol: Front Cardiovasc Med, 2021; 8; 789331
21.. Johnson JA, Caudle KE, Gong L, Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update: Clin Pharmacol Ther, 2017; 102(3); 397-404
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250