08 July 2022: Articles 
Clinical Outcomes of Sotrovimab Treatment in 10 High-Risk Patients with Mild COVID-19: A Case Series
Unusual or unexpected effect of treatment
Kenichiro Takeda1ABCDEF*, Hajime Kasai1ABCDEF, Seiichiro Sakao1ABCDEF, Mikihito Saito1ABCDE, Kohei Shikano1ABCDEF, Akira Naito1ABDE, Mitsuhiro Abe1BE, Takeshi Kawasaki1ABCDEF, Misuzu Yahaba2ABCDEF, Toshibumi Taniguchi3ABCDEF, Hidetoshi Igari2ABCDEF, Takuji Suzuki1ABCDEFDOI: 10.12659/AJCR.936832
Am J Case Rep 2022; 23:e936832
Abstract
BACKGROUND: Although sotrovimab reduces the risk of hospitalization or death due to COVID-19, there have been few reports of its use in clinical practice. Particularly, information on the effectiveness of sotrovimab against the omicron variant of the virus is limited. We present 10 cases of COVID-19 treated with sotrovimab at our unit between December 2021 and February 2022.
CASE REPORT: The age of the patients ranged from 32 to 81 years (median: 40 years). The comorbidities included lung cancer, cardiovascular disease, chronic kidney disease requiring hemodialysis, and AIDS. Two of the patients were also organ recipients. Oxygen saturation (SpO2) was above 97% in all patients. None of the patients presented with pneumonia on admission. However, blood test results showed that all patients had risk factors for severe COVID-19 outcomes. The interval from symptom onset to sotrovimab administration and resolution ranged from 2 to 5 days (median: 2 days) and 2 to 15 days (median: 5 days), respectively. Only 1 patient developed pneumonia and was treated with remdesivir after sotrovimab administration. However, this patient did not require oxygen therapy. Although no moderate to severe adverse events were observed, a mild adverse event was observed in 1 patient.
CONCLUSIONS: Sotrovimab could be safe and effective in preventing progression of COVID-19 in patients with a variety of underlying diseases and who are at high risk of severe disease outcomes.
Keywords: COVID-19, SARS-CoV-2 Variants, Sotrovimab, Adult, Aged, Aged, 80 and over, Antibodies, Monoclonal, Humanized, Antibodies, Neutralizing, Humans, Middle Aged, SARS-CoV-2
Background
Since December 2019, COVID-19 has spread rapidly worldwide. A variety of therapeutic agents against the disease have been developed, including neutralizing antibodies. Sotrovimab is a pan-sarbecovirus monoclonal antibody designed to prevent COVID-19 progression in patients at high risk when administered in the early disease course. Moreover, it has been shown to reduce the risk of hospitalization or death by 85% [1]. In Japan, sotrovimab was approved on September 27, 2021.
Since November 2021, the number of patients infected with the omicron variant of SARS-CoV-2, which gradually replaced the previously dominant delta variant, has been increasing rapidly [2]. In Japan, similar events occurred since December 2021. Although the omicron variant was shown to be resistant to neutralization by several therapeutic antibodies in vitro, there has been some evidence indicating that sotrovimab may be effective against this new variant [3]. Nevertheless, the available information on the effectiveness of sotrovimab against COVID-19 (including the omicron variant) in clinical practice is limited. In addition, the safety and efficacy of sotrovimab in patients with various comorbidities are unknown.
We present the clinical outcomes of sotrovimab treatment among 10 high-risk patients with mild COVID-19 at our hospital between December 2021 and February 2022.
Case Report
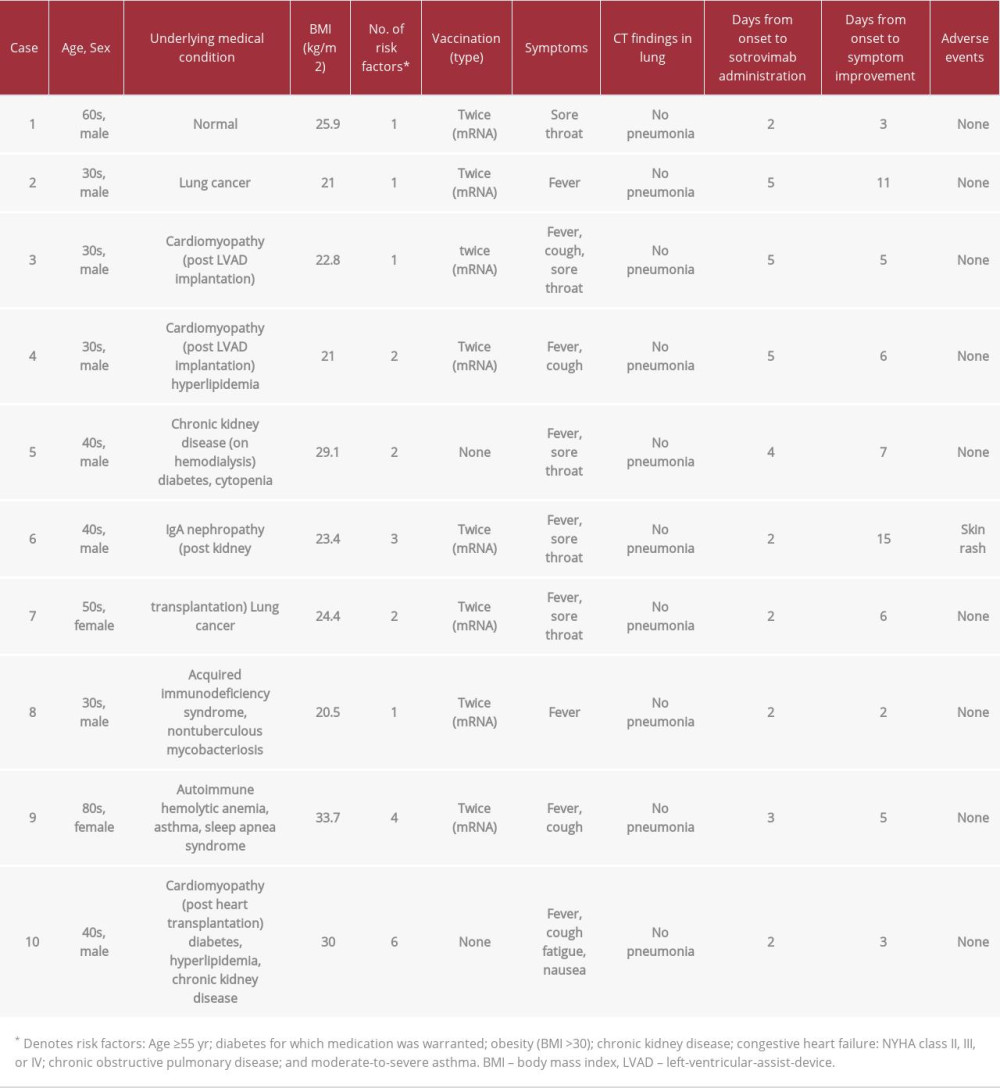
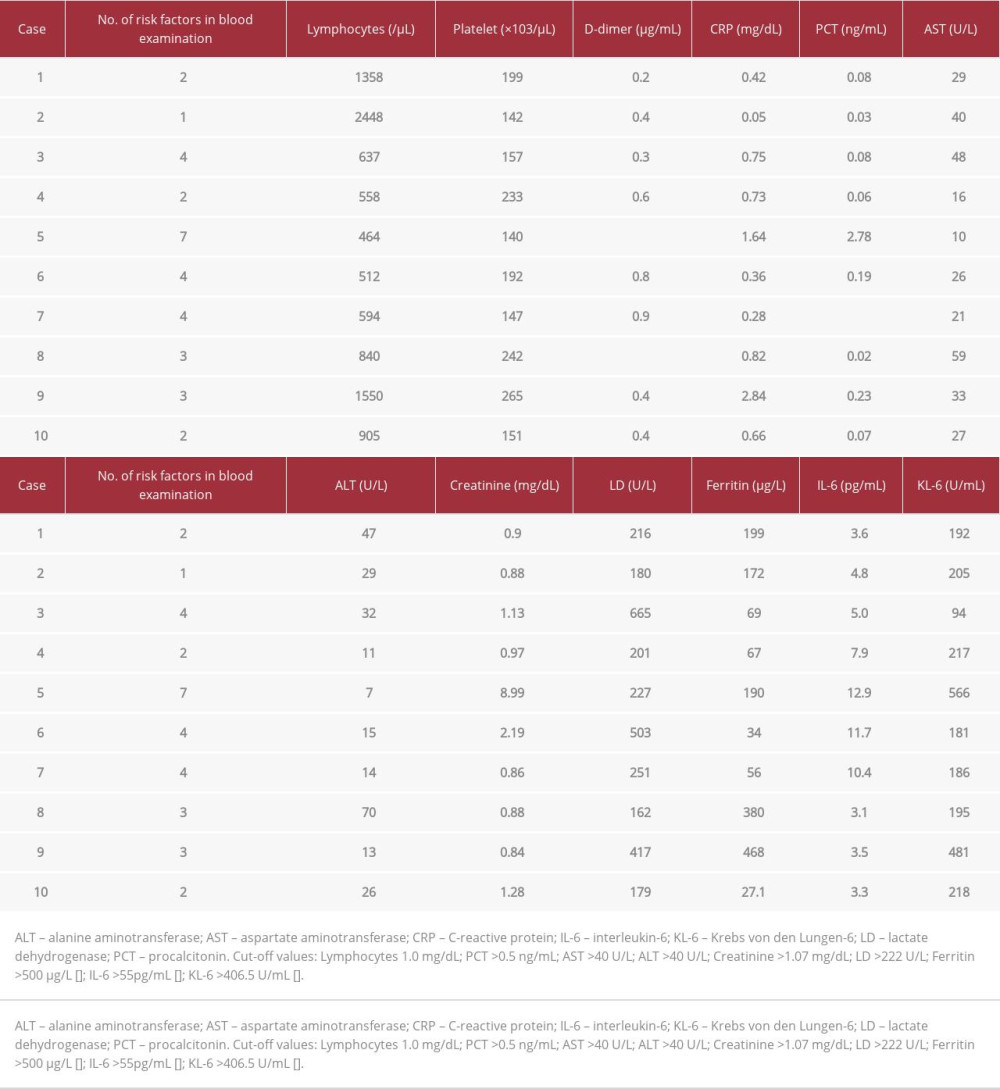
The demographic and clinical data of the 10 patients are presented in Table 1. Genome sequencing was performed in 2 patients and confirmed their infection with the omicron variant. The patients’ comorbidities included lung cancer (n=2, 20%), cardiovascular disease (n=2, 20%), chronic kidney disease (n=3, 30%), and AIDS (n=1, 10%). In addition, 2 of the patients (20%) had received an organ transplant. Although 8 of the 10 patients had received 2 doses of a vaccine against COVID-19, the remaining 2 were unvaccinated. On admission, fever was a common symptom in the patients (n=9, 90%). In addition, oxygen saturation (SpO2) was in the 97% to 100% range (median value: 97%) on room air. None of the patients had signs of pneumonia on computed tomography. The laboratory test results on admission are presented in Table 2. Blood examination revealed lymphopenia (n=8), elevated creatinine levels (n=4), and elevated lactate dehydrogenase levels (n=5), which are known risk factors for COVID-19 progression. Therefore, all patients were at a higher risk of developing severe disease outcomes according to these blood test results.
The interval from disease onset to sotrovimab administration and symptom resolution ranged between 2 and 5 days (median: 2 days) and between 2 and 15 days (median: 5 days), respectively. Adverse events were observed in only 1 patient (case 6) who developed skin rash, which was treated with topical steroids. The patient also developed pneumonia and received remdesivir. However he did not require oxygen therapy. The remaining patients also did not require any oxygen therapy. All patients were discharged after fulfilling the hospital discharge criteria in Japan [4]. There were no significant sequel-ae of COVID-19 in any of the patients.
Discussion
The 10 cases described herein highlight several noteworthy findings. First, sotrovimab may have effectively prevented progression in patients with a variety of comorbidities and infected by the omicron variant of SARS-CoV-2. Second, sotrovimab administration was safe, with no observed moderate or severe adverse events.
Neutralizing antibodies, including sotrovimab, are administered to patients with mild COVID-19 who have risk factors for developing a severe condition. In a phase 3 trial of sotrovimab, risk factors for progression to severe disease outcomes were defined. These risk factors include age above 55 years, obesity (body mass index >30), diabetes requiring medication, chronic kidney disease, congestive heart failure (NYHA class II, III, or IV), chronic obstructive pulmonary disease, and moderate-to-severe asthma. However, data from patients with renal or cardiac disease was limited [1]. Our case series included 3 cases of renal disease and 3 cases of cardiac disease. Furthermore, 2 of 10 patients were unvaccinated for COVID-19.
The risk factors associated with COVID-19 severity include malignancy [5], immunodeficiency due to organ transplantation [6], treatment with steroids [7] or anti-rheumatic drugs [8], and HIV infection with a CD4+ count below 200 cells/μL [9]. According to a meta-analysis of COVID-19 outcomes, lymphopenia, thrombocytopenia, and elevated levels of D-dimer, C-reactive protein, procalcitonin, creatine kinase, aspartate aminotransferase, alanine aminotransferase, creatinine, and lactate dehydrogenase were significantly associated with the need for mechanical ventilation and mortality [10]. In the present case series, all patients exhibited at least 1 of these above-mentioned risk factors and therefore had a higher risk of developing severe COVID-19. Only 1 of the patients, who had undergone kidney transplantation, developed pneumonia and was treated with remdesivir. However, the symptoms did not worsen in the remaining 9 patients (90%). None of the patients developed a severe condition, suggesting that sotrovimab was effective in preventing progress to this stage even in patients with multiple risk factors.
In this case series, there were no moderate-to-severe adverse events. Only 1 patient (case 6) developed skin rash that may have been caused by the concomitant use of acetaminophen or resulted from viral infection. Based on these results, it can be concluded that sotrovimab could be safely administered to immunosuppressed patients or those with underlying diseases such as heart failure or chronic kidney disease.
This case series includes a relatively small number of cases. In addition, the omicron variant of SARS-CoV-2 has been reported to cause a less severe disease compared with other variants [11], which may be the reason why there were no severe cases in our patient cohort. Furthermore, previous vaccination may have contributed to prevent disease progression. Studies including additional cases from clinical practice are needed to confirm the efficacy of sotrovimab in preventing disease progression into a severe condition in patients presenting with various comorbidities.
Conclusions
Phase 3 trials have provided limited data on the efficacy and safety of sotrovimab in patients with severe comorbidities such as cancer, cardiac disease, renal disease, and immunocompromised conditions. We presented the clinical outcomes of sotrovimab treatment in 10 high-risk patients with mild COVID-19 at our hospital between December 2021 and February 2022. The clinical outcomes suggest that sotrovimab might be safe and effective in preventing disease progression in COVID-19 patients with several different comorbidities.
References:
1.. Gupta A, Gonzalez-Rojas Y, Juarez E, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab: N Engl J Med, 2021; 385; 1941-50
2.. , COVID-19 Weekly Epidemiological Update Edition 76 January, 2022 [cited 2022 March 16]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---25-january-2022
3.. Hoffmann M, Kruger N, Schulz S, The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic: Cell, 2022; 185; 447-56.e11
4.. , Information for medical institutions (treatment guidelines, clinical research, etc.), 2022 [cited 2022 March 16]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00111.html
5.. Liang W, Guan W, Chen R, Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China: Lancet Oncol, 2020; 21; 335-37
6.. Latif F, Farr MA, Clerkin KJ, Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019: JAMA Cardiol, 2020; 5; 1165-69
7.. Brenner EJ, Ungaro RC, Gearry RB, Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry: Gastroenterology, 2020; 159; 481-91 e3
8.. Michelena X, Borrell H, Lopez-Corbeto M, Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs: Semin Arthritis Rheum, 2020; 50; 564-70
9.. Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR, Characteristics and outcomes of COVID-19 in patients with HIV: A multicentre research network study: AIDS, 2020; 34; F3-8
10.. Malik P, Patel U, Mehta D, Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis: BMJ Evid Based Med, 2021; 26; 107-8
11.. Wolter N, Jassat W, Walaza S, Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study: Lancet, 2022; 399; 437-46
12.. Gandini O, Criniti A, Ballesio L, Serum Ferritin is an independent risk factor for acute respiratory distress syndrome in COVID-19: J Infect, 2020; 81; 979-97
13.. Aziz M, Fatima R, Assaly R, Elevated interleukin-6 and severe COVID-19: A meta-analysis: J Med Virol, 2020; 92; 2283-85
14.. d’Alessandro M, Cameli P, Refini RM, Serum KL-6 concentrations as a novel biomarker of severe COVID-19: J Med Virol, 2020; 92; 2216-20
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250