07 July 2022: Articles 
A 25-Year-Old Saudi Woman with a 2-Year History of Antisynthetase Syndrome with Interstitial Lung Disease Who Commenced Azathioprine Treatment in the Third Trimester of Pregnancy and Had a Successful Birth at Term
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Lama A. Alshwairikh1ABEF*, Zeneb Babay1ADOI: 10.12659/AJCR.936833
Am J Case Rep 2022; 23:e936833
Abstract
BACKGROUND: Antisynthetase syndrome (ASS) is a rare systemic autoimmune disease. The clinical features of ASS include interstitial lung disease (ILD), myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and unexplained fever. There is a paucity of reported cases and management guidelines in pregnancy. This report describes the case of a 25-year-old Saudi woman with a 2-year history of ASS with ILD who commenced azathioprine treatment in the third trimester and had a successful birth at term.
CASE REPORT: A 25-year-old Saudi primigravida woman with a 2-year history of ASS with ILD presented at 26 weeks of gestation after being lost to prepregnancy follow-up and discontinuing her medications. Azathioprine treatment was commenced, and despite poor prepregnancy follow-up, her pregnancy remained uneventful until 39 weeks, when fetal ultrasonography showed oligohydramnios. Therefore, labor induction was initiated, and she delivered vaginally with no postpartum complications or flare-ups.
CONCLUSIONS: The multisystem autoimmune disease ASS is a rare condition, and there are no clinical guidelines for its management in pregnant women. This case report highlights some aspects of ASS management and the importance of a multidisciplinary approach.
Keywords: Anti-Jo-1 Autoantibody, Antisynthetase Syndrome, Pregnancy, myositis, cryptogenic organizing pneumonia, Adult, Autoantibodies, Autoimmune Diseases, azathioprine, Female, Humans, Lung Diseases, Interstitial, Pregnancy Trimester, Third, Saudi Arabia
Background
Antisynthetase syndrome (ASS) is a rare systemic autoimmune disease with an estimated prevalence of 1 to 9 per 100 000 [1,2]. ASS is characterized by autoantibodies against aminoacyl transfer ribonucleic acid synthetases (tRNAs) [3–5]. The most identified tRNA autoantibody is anti-histidyl (Jo-1), a myositis-specific antibody [3–5]; others include anti-threonyl (anti-PL7) and anti-alanyl (anti-PL12) [3–5]. Publications report that ASS symptoms and morbidity vary depending on the identified antibodies [6,7]. For instance, anti-Sjögren’s syndrome-related antigen A (anti-Ro/SSA), a myositis-associated antibody [8], has been associated with ASS and a predisposition to severe ILD and lung fibrosis [9,10].
ASS diagnosis is challenging due to symptom heterogeneity and similarity to those of other connective tissue diseases and idiopathic inflammatory myopathies [11,12]. Clinical features include ILD, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and unexplained fever [3,11]. Pulmonary hypertension is also associated with ASS [11,12]. Two diagnostic criteria have been proposed by Solomon el al and Connors et al (Table 1) [13,14]. No unified treatment protocol exists for ASS [11], and the systems involved and symptom severity are considered when planning the management [11]. The typical first-line treatment is corticosteroids; however, when corticosteroids are used as monotherapy, lung disease frequently recurs with tapering [1,11,12]. Other agents used as adjunct and maintenance treatments include azathioprine, mycophenolate mofetil, tacrolimus, cyclosporine, cyclophosphamide, and rituximab [1,11,12]. When the symptoms improve and the disease stabilizes, corticosteroids are often gradually discontinued [12]. For treatment in pregnancy, there are no management guidelines, and the literature is scarce [15–18]. This report describes the case of a 25-year-old Saudi woman with a 2-year history of ASS with ILD who commenced azathioprine treatment in the third trimester and had a successful birth at term despite poor follow-up.
Case Report
A 25-year-old Saudi primigravida woman at 26 weeks of gestation presented to the pulmonology clinic for a follow-up. She had a past medical history of ASS with ILD and no surgical history. She had no allergies and no history of blood transfusion, smoking, or alcohol or substance use. Her medications included oral ferrous sulfate-folic acid 150-0.5 mg and oral calcium carbonate 600 mg daily.
She was diagnosed with ASS at the age of 23. At that time, she presented to the pulmonology clinic with 2 months of shortness of breath, nonproductive cough, arthritis, and mechanic’s hands. She had no past medical history or exposure to occupational or environmental agents. Investigations showed positive antinuclear antibody (ANA) and anti-Ro/SSA. Salivary gland/lip biopsy samples for autoimmune sialadenitis/Sjogren’s syndrome, anti-smooth muscle antibody (ASMA), myeloperoxidase anti-neutrophil cytoplasmic (MPO), and ribonucleo-protein (RNP) antibodies were negative. Pulmonary function test (PFT) results showed a restrictive lung pattern (Table 2). Echocardiography was normal. High-resolution computed tomography (HRCT) showed peripheral lower lobe predominant interlobular septal thickening with ground-glass attenuation and traction bronchiectasis with no evidence of honeycombing, consistent with combined nonspecific interstitial pneumonia (NSIP)-organizing pneumonia (OP) (Figure 1). A transbronchial biopsy sample from the left lower lobe showed chronic inflammatory cell infiltration and early Masson’s body formation consistent with secondary cryptogenic OP. ILD (NSIP-OP) was diagnosed based on symptoms, PFT, HRCT, and transbronchial biopsy. ASS was diagnosed according to Solomon’s and Connor’s criteria (Table 1) [13,14]. Her daily treatment included oral prednisolone 25 mg, oral trimethoprim/sulfamethoxazole 400 mg/80 mg, and oral mycophenolate mofetil 2 g, and she did not require supplemental oxygen. At the 3-month follow-up, her symptoms had improved, but her PFT results had not (Table 2). Accordingly, rituximab was added (1 g intravenous infusion every 2 weeks for 1 month, then 1 dose every 6 months). Three months later, PFT results improved (Table 2), but HRCT showed no significant change from the previous study (Figure 2). At the 18-month follow-up, PFT results showed further improvement (Table 2), although HRCT revealed no significant change (Figure 3). As she was planning pregnancy, she was advised to discontinue mycophenolate mofetil, taper prednisolone to 5 mg, commence azathioprine, and return after 1 month.
Unfortunately, she was lost to follow-up. Then, at 26 weeks of gestation, she presented to the pulmonology clinic and had stopped taking all the medications 2 months before conception. She had mild shortness of breath but no other respiratory symptoms, myositis, or arthritis. Oral azathioprine 50 mg daily was initiated with the goal of increasing the dose to 100 mg. When she returned for a follow-up after 1 week, her complete blood count (CBC) revealed a normal white blood cell (WBC) count and low hemoglobin (7.5 g/dL). She received 6 doses of 200 mg of intravenous iron sucrose, and she was referred to the obstetric maternal-fetal medicine clinic.
At her obstetric booking visit at 29 weeks of gestation, she reported an improvement in her shortness of breath. Fetal ultrasonography showed normal anatomy and heart rate, and weight was estimated to be within the normal range. Her WBC count was normal, her hemoglobin level increased to 9.6 g/dL, and her oral glucose challenge test result was normal. Fetal echocardiogram was ordered due to the presence of anti-Ro/ SSA, but, unfortunately, she missed that appointment. After 1 week, a multidisciplinary meeting with her pulmonologist took place to establish a management plan. The impression was that her disease was stable, it would not affect the timing and mode of delivery, and the shortness of breath was likely due to low hemoglobin. Azathioprine was increased to 100 mg. Although she was not followed up regularly, she was compliant with azathioprine. There was no concern for severe pulmonary impairment, and a repeat PFT was not performed, as she was asymptomatic and did not require supplemental oxygen.
Her pregnancy was uneventful until she presented to the Emergency Department at 39 weeks of gestation due to decreased fetal movement for 2 days. She had no labor pain or vaginal leakage. Her vital signs were within the normal range, and fetal heart tracing by cardiotocography was category 1. Fetal ultrasonography showed oligohydramnios (amniotic fluid index 3.1), and the estimated fetal weight was within the normal range. Due to oligohydramnios, labor was induced by dinoprostone 10 mg vaginal insert, and labor progressed smoothly. She received epidural anesthesia and had a vacuum-assisted vaginal delivery due to poor maternal effort. She delivered a girl with an Apgar score of 8 and 9 at 1 and 5 minutes, respectively, and the cord pH was 7.26. The patient and her baby were discharged home on day 2 post-partum in good condition.
At 2-month follow-up, she was asymptomatic and compliant with azathioprine. She was advised to continue follow-up with her pulmonologist and present for preconception counseling before planning subsequent pregnancies.
Discussion
Although our patient stopped her preconception treatment without consultation, conceived only 2 months after mycophenolate mofetil withdrawal, and was not adherent to her follow-up, she had good outcomes without maternal or fetal morbidities other than oligohydramnios. This case highlights some aspects of optimal ASS management in pregnancy that our patient missed.
The effect of ASS on pregnancy and management has been extrapolated from other connective tissue and autoimmune disorders [15]. The possible morbidities include preeclampsia, fetal growth restriction, prematurity, congenital heart block, and neonatal lupus in the presence of anti-SSA/Ro or anti-Sjögren’s syndrome-related antigen B (anti-SSB/La) [19–22]. The disease course may partly depend on the prepregnancy status, as evidenced by previous publications [15–18]. Similar to our patient, a patient with stable disease preconception who was on azathioprine and prednisone had an uncomplicated pregnancy and term delivery [15]. Another successful outcome was reported for a patient with severe ILD and fibrosis who was on long-term rituximab infusion for 5 years and conceived 2 years after clinical condition improvement and treatment discontinuation [16]. Adverse outcomes were described in patients with severe disease. One patient had severe myositis and was newly diagnosed in her second trimester, but her pregnancy ended with a miscarriage [17]. Another patient with pulmonary hypertension and substantial parenchymal restrictive disease prior to conception had worsening PFT results and required supplemental oxygen during pregnancy. She had an iatrogenic preterm delivery at 35 weeks of gestation due to oligohydramnios attributed to chronic maternal hypoxemia [18]. Although our patient developed oligohydramnios, it was unlikely due to chronic maternal hypoxemia, as her disease was quiescent.
Management in pregnancy starts with a preconception visit to plan conception when the disease is in remission, discontinue teratogenic medications, and perform baseline PFT and echocardiography [11,12,15]. Antepartum, low-dose aspirin is initiated at between 12 and 28 weeks of gestation (optimally before 16 weeks) as recommended by the American College of Obstetricians and Gynecologists-Society for Maternal-Fetal Medicine and the National Institute for Health and Care Excellence guidelines for pregnant women with autoimmune disorders to decrease the risk of preeclampsia [23,24]. Moreover, serial fetal growth assessment should be considered, and fetal echocardiography should be done to assess congenital heart block in patients with anti-Ro/SSA or anti-SSB/La [21,22]. For treatment in pregnancy, corticosteroids, azathioprine, and cyclosporine are safely used in pregnant women with systemic lupus erythematosus [25–27]; cyclophosphamide is contraindicated due to the increased risk of miscarriage and congenital anomalies [25–27]; and because data on rituximab are limited, rituximab discontinuation before conception is advised [25–27]. Tacrolimus and cyclosporine are safe options for pregnant women after organ transplantation; however, mycophenolate mofetil is contraindicated, as it is associated with a high risk of miscarriage and congenital anomalies and must be discontinued 3–6 months before pregnancy [28,29]. As the course of ASS is unknown, a multidisciplinary approach is advised, particularly if severe manifestations such as pulmonary hypertension or severe ILD develop to alter the treatment and timing of delivery.
Conclusions
The multisystem autoimmune disease ASS is a rare condition, and there are no clinical guidelines for its management in pregnant women. This case highlights some aspects of ASS management in pregnancy and the importance of a multidisciplinary approach.
Figures
References:
1.. Opinc AH, Makowska JS, Antisynthetase syndrome – much more than just a myopathy: Semin Arthritis Rheum, 2021; 51(1); 72-83
2.. Imbert-Masseau A, Hamidou M, Agard C, Antisynthetase syndrome: Joint Bone Spine, 2003; 70(3); 161-68
3.. Katzap E, Barilla-LaBarca ML, Marder G, Antisynthetase syndrome: Curr Rheumatol Rep, 2011; 13(3); 175-81
4.. Bauhammer J, Fiehn C, Antisynthetase syndromes.]: Z Rheumatol, 2019; 78(7); 645-55 [in German]
5.. Cojocaru M, Cojocaru IM, Chicos B, New insights into antisynthetase syndrome: Maedica (Bucur), 2016; 11(2); 130-35
6.. Betteridge Z, McHugh N, Myositis-specific autoantibodies: an important tool to support diagnosis of myositis: J Intern Med, 2016; 280(1); 8-23
7.. Marie I, Hatron PY, Cherin P, Functional outcome and prognostic factors in anti-Jo1 patients with antisynthetase syndrome: Arthritis Res Ther, 2013; 15(5); R149
8.. Ghirardello A, Borella E, Beggio M, Myositis autoantibodies and clinical phenotypes: Auto Immun Highlights Aug; 5(3); 69-75
9.. La Corte R, Lo Mo Naco A, Locaputo A, In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease: Autoimmunity, 2006; 39(3); 249-53
10.. Mileti LM, Strek ME, Niewold TB, Clinical characteristics of patients with anti-Jo-1 antibodies: A single center experience: J Clin Rheumatol, 2009; 15(5); 254-55
11.. Marco JL, Collins BF, Clinical manifestations and treatment of antisynthetase syndrome: Best Pract Res Clin Rheumatol, 2020; 34(4); 101503
12.. Witt LJ, Curran JJ, Strek ME, The diagnosis and treatment of antisynthetase syndrome: Clin Pulm Med, 2016; 23(5); 218-26
13.. Solomon J, Swigris JJ, Brown KK, Myositis-related interstitial lung disease and antisynthetase syndrome: J Bras Pneumol, 2011; 37(1); 100-9
14.. Connors GR, Christopher-Stine L, Oddis CV, Danoff SK, Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years?: Chest Dec, 2010; 138(6); 1464-74
15.. Green LJ, O’Neill L, Frise CJ, Antisynthetase syndrome in pregnancy: A case and review of the literature: Obstet Med, 2020; 13(2); 96-100
16.. Dalmau-Carolà J, Childbirth in a woman with antisynthetase syndrome and severe lung disease on long-term rituximab therapy: Indian J Rheumatol, 2016; 11(2); 117-18
17.. Satoh M, Ajmani AK, Hirakata M, Onset of polymyositis with auto-antibodies to threonyl-tRNA synthetase during pregnancy: J Rheumatol, 1994; 21(8); 1564-66
18.. Dumitrascu CI, Olsen DA, Arendt KW, Antisynthetase syndrome with severe interstitial lung disease in pregnancy: Case Rep Anesthesiol, 2021; 2021; 1150394
19.. Ateka-Barrutia O, Nelson-Piercy C, Connective tissue disease in pregnancy: Clin Med (Lond), 2013; 13(6); 580-84
20.. Marder W, Littlejohn EA, Somers EC, Pregnancy and autoimmune connective tissue diseases: Best Pract Res Clin Rheumatol, 2016; 30(1); 63-80
21.. Tunks RD, Clowse ME, Miller SG, Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents: Am J Obstet Gynecol, 2013; 208(1); 64.e1-7
22.. Brucato A, Frassi M, Franceschini F, Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women: Arthritis Rheum, 2001; 44(8); 1832-35
23.. , Low-dose aspirin use for the prevention of preeclampsia and related morbidity and mortality Available from: [Accessed 16 February 2022]https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/12/low-dose-aspirin-use-for-the-prevention-of-preeclampsia-and-related-morbidity-and-mortality
24.. , Reducing the risk of hypertensive disorders in pregnancy Available from: [Accessed 16 February 2022]https://wwwniceorg.uk/guidance/ng133/chapter/recommendations#reducing-the-risk-of-hypertensive-disorders-in-pregnancy
25.. Lateef A, Petri M, Managing lupus patients during pregnancy: Best Pract Res Clin Rheumatol, 2013; 27(3); 435-47
26.. Ponticelli C, Moroni G, Immunosuppression in pregnant women with systemic lupus erythematosus: Expert Rev Clin Immunol, 2015; 11(5); 549-52
27.. Cimpoca BA, Nedelea F, Furtuna M, Managing Crohn’s disease during pregnancy: Maedica (Bucur), 2016; 11(3); 221-26
28.. Boulay H, Mazaud-Guittot S, Supervielle J, Maternal, foetal and child consequences of immunosuppressive drugs during pregnancy in women with organ transplant: A review: Clin Kidney J, 2021; 14(8); 1871-78
29.. Deshpande NA, Coscia LA, Gomez-Lobo V, Pregnancy after solid organ transplantation: A guide for obstetric management: Rev Obstet Gynecol, 2013; 6(3–4); 116-25
Figures
Tables
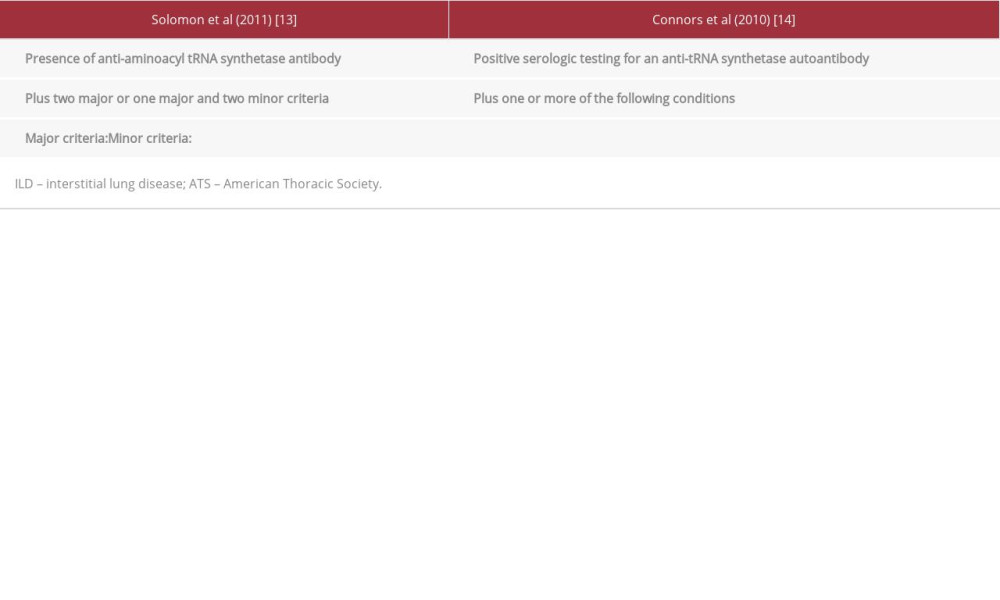 Table 1.. Proposed criteria for the diagnosis of antisynthetase syndrome by Solomon et al [13] and Conors et al [14].
Table 1.. Proposed criteria for the diagnosis of antisynthetase syndrome by Solomon et al [13] and Conors et al [14].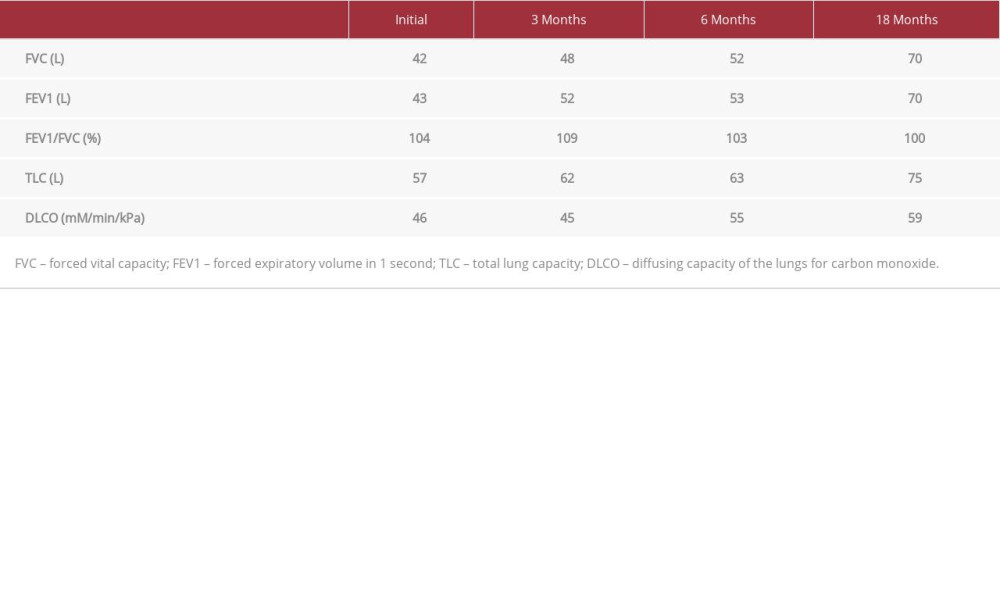 Table 2.. Pulmonary functions test results for the patient at first presentation and at follow-ups.
Table 2.. Pulmonary functions test results for the patient at first presentation and at follow-ups. Table 1.. Proposed criteria for the diagnosis of antisynthetase syndrome by Solomon et al [13] and Conors et al [14].
Table 1.. Proposed criteria for the diagnosis of antisynthetase syndrome by Solomon et al [13] and Conors et al [14]. Table 2.. Pulmonary functions test results for the patient at first presentation and at follow-ups.
Table 2.. Pulmonary functions test results for the patient at first presentation and at follow-ups. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








