10 August 2022: Articles 
Erasmus Syndrome: A Case Report and Literature Review
Rare disease
Jan Michael Jesse Lomanta1ABCDEF*, Mary Antonette Atienza2BDE, Juan Raphael M. Gonzales3DEF, Eric Jason Bautista Amante3DE, Sheen C. Urquiza4DEF, Hanna Lucero-Orillaza2DE, Joel Marquez Santiaguel15ADGDOI: 10.12659/AJCR.937061
Am J Case Rep 2022; 23:e937061
Abstract
BACKGROUND: Erasmus syndrome is a rare disease entity characterized by the development of systemic sclerosis (SSc) in a background of silica exposure or silicosis.
CASE REPORT: We report the case of a 40-year-old Filipino man who previously worked in a silica grind mill for 10 years and eventually developed Erasmus syndrome. The patient initially presented with chronic back pain in 2018 associated with findings of pulmonary tuberculosis on chest X-ray, with notable improvement after 6 months of anti-tuberculosis treatment. However, his back pain recurred in 2021; this time with arthralgia, Raynaud’s phenomenon, thickening of both hands, skin hypopigmentation on the chest, back, and forehead, and exertional dyspnea. Physical examination revealed salt-and-pepper dermopathy and skin tightening over the back, chest, and extremities. Mobility of his hands was limited, associated with sclerodactyly and digital pitting. Antinuclear antibody-immunofluorescence and anti-scleroderma-70 antibodies were strongly positive, confirming the diagnosis of SSc. Chest computed tomography illustrated multiple subcentimeter nodules and enlarged mediastinal lymph nodes with eggshell calcifications, consistent with silicosis. Spirometry with body plethysmography was normal but diffusing capacity for carbon monoxide was severely reduced. Histopathology of the skin showed markedly thickened collagen bundles in the dermis.
CONCLUSIONS: Chronic silica exposure is a risk factor for the development of silicosis. The clinical course of patients with silicosis may be complicated by SSc. Maintaining a high index of suspicion is key to the diagnosis of Erasmus syndrome. The present report emphasizes the importance of preventive measures and surveillance among those with occupational exposure to silica. To our knowledge, this is the first documented case of Erasmus syndrome in the Philippines.
Keywords: Autoimmune Diseases, Occupational Exposure, Scleroderma, Systemic, Silicosis, Adult, Antibodies, Antinuclear, Humans, Raynaud Disease, Silicon Dioxide, Syndrome
Background
Silicosis is a preventable fibrosing lung disease caused by inhalation of crystalline silica [1,2]. The earliest documented records demonstrating the association between silica exposure and the development of respiratory disorders date back to the 1690s [2]. It was Visconti who coined the term “silicosis” in 1870 to describe the lung diseases caused by respirable silica [2]. Despite being among the oldest known occupational lung diseases, existing epidemiologic data on the global incidence and prevalence of silicosis seems outdated. An earlier report by Rosenman et al (2003) based on United States (US) death certificates, the Michigan state surveillance system, and capture-recapture analysis estimated that between 1987 and 1996, there were approximately 3600–7000 cases of silicosis reported each year [3]. During this decade, roughly 3000 deaths were attributed to silicosis in the US alone [3]. Although mortality from silicosis has decreased substantially, the medical and economic burden of silica exposure remains high. An analysis of health insurance claims from US Medicare beneficiaries between 1999 and 2014 revealed that more than 20 000 cases met the silicosis case definition [4].
Silica, which occurs in both amorphous and crystalline forms, is among the most abundant minerals on earth [5]. It is found in mineral ores, sand, and rocks such as sandstone, granite, and quartz [2]. The crystalline form of silica, when inhaled, is the form implicated in the development of occupational lung diseases [2,5,6]. An occupation that disturbs the earth’s crust, such as processing silica-containing rock, is a risk factor for acquiring silicosis. These major environmental exposures include but are not limited to sandblasting, mining, stone cutting, granite quarrying, and silica flour packing [7,8]. When silica dusts are inhaled, mineral deposits lodge at the level of the terminal bronchioles and alveoli [9]. The presence of foreign material in the lungs leads to a cascade of inflammatory events such as macrophage activation, release of inflammatory cytokines, generation of free radicals, and upregulation of cell-signaling pathways leading to symptoms such as dyspnea, cough, and easy fatigability [9].
Apart from silicosis, silica exposure has also been associated with a number of systemic autoimmune diseases [5]. A literature review conducted by Miller et al (2012) provided epidemiologic evidence linking silica inhalation to the development of systemic lupus erythematosus, rheumatoid arthritis, primary systemic vasculitis, Wegener’s granulomatosis, and systemic sclerosis (SSc) [10]. These findings were consistent with a systematic review by Shtraichman and colleagues (2015) on the rheumatologic complications of patients with silica-related lung diseases treated in a lung transplant center in Israel between 1997 and 2012 [11]. Forty patients with silicosis were identified and 9 developed autoimmune diseases: 3 were diagnosed with SSc; 2 had rheumatoid arthritis; 2 were confirmed to have mixed connective tissue disease; 1 had Sjogren’s syndrome; and 1 had polymyositis syndrome [11]. The presence of SSc in patients with silicosis or silica exposure has been termed Erasmus syndrome. This condition is rare. In fact, a recent retrospective study on a large Brazilian cohort estimated the prevalence of Erasmus syndrome to be only 0.9% among SSc patients [12].
Here, we present the case of a Filipino male who worked in a silica mill abroad for 10 years and eventually developed debilitating silicosis and SSc. To our knowledge, this is the first documented case of Erasmus syndrome reported in the Philippines.
Case Report
This is the case of a 40-year-old Filipino male, non-smoker, with no known comorbidities, who worked as a stone crusher operator in a silica grind mill in Saudi Arabia from 2002 to 2012. The patient inconsistently wore coveralls and face mask as protective gear at work depending on the availability of supply. The history of illness started in 2018 when he complained of recurrent upper back pain characterized as stiffening of the muscles, occurring throughout the day, which was partially relieved by paracetamol. He consulted at a private clinic where a chest X-ray was done showing diffused nodular opacities in a miliary pattern, but with a negative sputum tuberculosis (TB) test (GeneXpert MTB/RIF sputum test). The patient was then treated as a case of clinically diagnosed pulmonary TB, completing 6 months of anti-TB regimen with subsequent improvement of the back pain.
In July 2021, the back pain recurred, this time accompanied by unintentional weight loss, exertional dyspnea, difficulty raising bilateral arms, and arthralgia of the wrists and knees. He started experiencing episodic changes in the color of his digits from white to red with self-resolution. He also had generalized darkening of the skin, hypopigmentation over the back and chest, and induration and thickening of both of his hands. Repeat sputum TB GeneXpert was negative but his chest X-ray again indicated miliary tuberculosis. He was treated with anti-TB medications for another 6 months but this did not improve his symptoms.
In February 2022, the patient sought consultation at a local hospital due to the persistence of his symptoms. A chest computed tomography (CT) scan was done showing multiple centrilobular nodules and short linear densities compatible with silicosis. He was then referred to our institution for further management.
Physical examination revealed salt-and-pepper dermopathy on the forehead, chest, nape, upper and lower back, and shins (Figure 1). The skin over the chest, back, and extremities was tight and shiny, with a modified Rodnan score of 19. His hands showed sclerodactyly (Figure 2A), digital pitted scars (Figure 2B), and limited mobility. Capillaroscopy showed few giant capillaries, no evident capillary loss, and a well-preserved regular distribution of capillaries (Figure 3). Pulmonary examination revealed good air entry, equal chest expansion, and clear breath sounds. Cardiac evaluation showed normal heart sounds, normal heart rate, and regular rhythm. Borg dyspnea score was moderate (3). The patient was able to cover 309 meters during the 6-minute walk test.
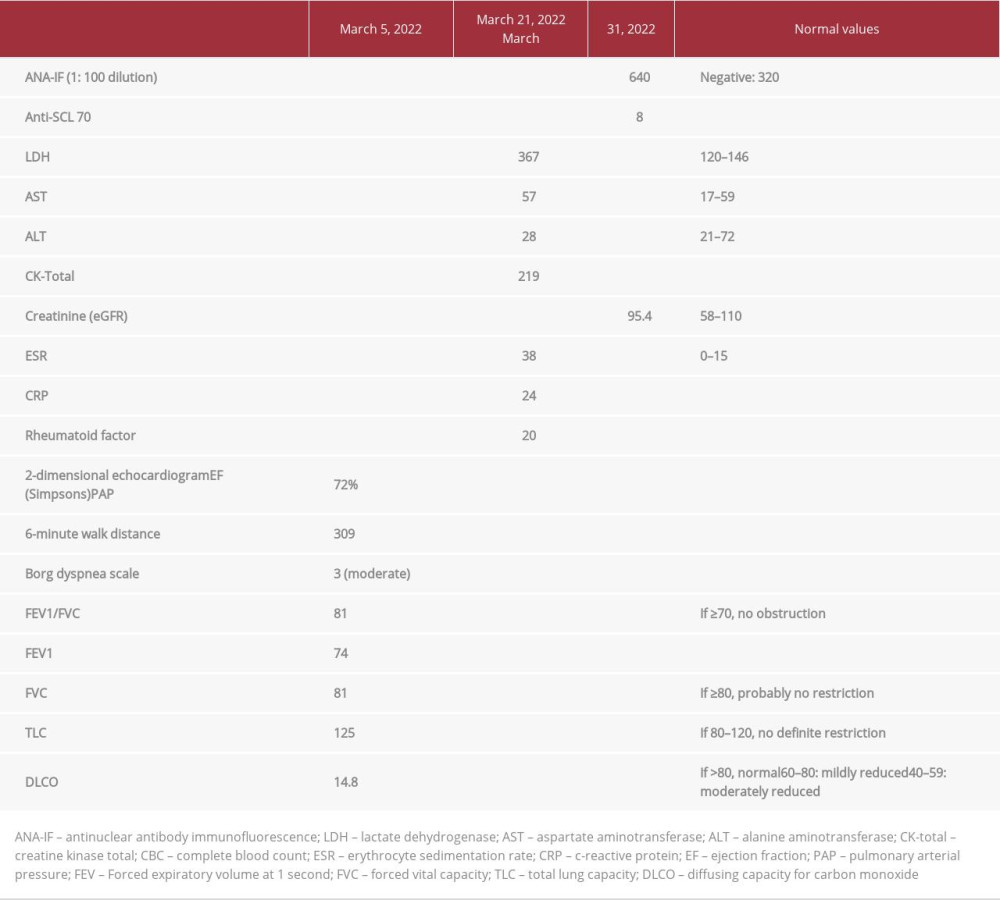
Complete blood count, creatine phosphokinase, lactate dehydrogenase, and creatinine were within normal limits. ANA-IF and anti-scleroderma-70 (Scl-70) antibodies were strongly positive (Table 1). Spirometry with body plethysmography was unremarkable but diffusing capacity for carbon monoxide (DLCO) was severely reduced at 14.8% predicted (Table 1). Echocardiogram showed an ejection fraction of 60%; pulmonary arterial pressure was normal. Chest X-ray showed multiple subcentimeter round nodules in the middle to upper lung fields measuring less than 3 millimeters (mm) in size (Figure 4). The patient’s silicosis was designated as “p”, according to the International Labor Office standardized radiographic classification for silicosis grading. Chest CT scan confirmed the presence of the nodules (Figure 5) and exhibited enlarged mediastinal lymph nodes with eggshell calcification (Figure 6). Skin biopsy demonstrated thickening of collagen bundles involving almost the entire dermis, which is typical of SSc (Figure 7). Based on the history of exposure, imaging findings consistent with silicosis, and clinical manifestations, and immunologic confirmation of SSc, the patient was diagnosed with Erasmus syndrome. He was then started on mycopheno-late mofetil 500 mg/tablet 3 times per day for the SSc and nifedipine 10 mg/tablet once per day for the Raynaud’s phenomenon. The patient was also referred to rehabilitation medicine.
Discussion
Silicosis is the most common form of pneumoconiosis, a group of interstitial lung diseases caused by inhalation of certain types of mineral dust [13,14]. Silicosis is specifically caused by inhalation and retention of crystalline silica in the lungs, leading to chronic inflammation and eventual lung fibrosis [5]. A detailed review on the pathogenesis of silica-induced lung diseases by Davis (1986) showed that the interaction between silica dusts and alveolar macrophages is a necessary prerequisite to the development of silicosis. Silica particles that are ingested by the resident and recruited alveolar macrophages in the lungs stimulate the secretion of inflammatory mediators such as interleukin-1 (IL-1), IL-2, and tumor necrosis factor (TNF) [15]. These proinflammatory mediators in turn activate T-helper cells and further recruit activated macrophages, to amplify the body’s inflammatory response. Activated macrophages and T lymphocytes both contribute to unregulated fibroblast proliferation and collagen deposition, causing lung fibrosis [15]. Silicosis may be classified as chronic, accelerated, or acute depending on how soon the patient develops symptoms from the time of exposure [2]. Symptoms include dry cough, exertional dyspnea, easy fatigability, pleuritic chest pain, and weight loss. Pathologically, silicosis may be categorized as nodular, silico-proteinosis, progressive massive fibrosis, or diffused interstitial fibrosis [2]. Because its respiratory manifestations and imaging findings can be easily confused with TB, an important differential to rule out among patients with silica exposure is active pulmonary TB. In fact, studies have suggested that silica exposure is a risk factor for the development of TB [16,17,18]. A meta-analysis conducted by Ehrlich et al (2021) illustrated an increased likelihood of developing TB among those with occupational exposure to silica dust (relative risk of 4.01, 95% confidence interval 2.88–5.58) [17].
SSc is a chronic autoimmune condition characterized by autoantibody production, small vessel vasculopathy, and fibro-blast dysfunction causing uncontrolled deposition of extra-cellular matrix [19,20,21]. Epidemiologic data have indicated that SSc has a prevalence of approximately 20 per million per year, with a strong predilection to women (~7: 1) [22]. SSc can affect virtually any organ. Typical manifestations include Raynaud’s phenomenon, telangiectasias, subcutaneous calcinosis, fibrosis and thickening of the skin, myalgia, esophageal dysmotility, pulmonary hypertension, and interstitial lung disease [23]. Despite the heterogeneity of the organ complications and the rate of progression, the presence of thick and indurated skin remains the cardinal feature of SSc. Interestingly, about 10% of SSc patients may have SSc-like organ involvement in the absence of the typical skin findings (scleroderma sine scleroderma). Our patient presented with the diffused type of scleroderma due to widespread skin thickening of the distal to the proximal extremities and the trunk, in the background of markedly elevated anti-Scl70 antibody levels [24]. Raynaud’s phenomenon also preceded the appearance of other manifestations. Although not present, kidney injury may also develop early in this subset of patients [25].
The exact pathophysiology of SSc is yet to be established, but chronic inflammation and immune dysregulation play crucial roles in its development [23]. Evidence indicates that certain environmental triggers acting on a genetically susceptible individual can perpetuate a state of chronic inflammation which may lead to SSc [26]. Environmental exposures such as bacteria, viruses, and toxins may induce epigenetic mutations that influence the expression of SSc [26].
The occurrence of silica-associated SSc was first documented in 1957 by a South African physician named Dr. LD Erasmus. Erasmus (1957) described 17 gold miners from Witwatersrand South Africa who developed Raynaud’s phenomenon, flexure contractures on the hands, resorption of the terminal phalanges in some cases, skin changes, and exertional dyspnea [46]. Of these 17 miners, 10 had no radiographic evidence of silicosis, 4 were identified to have probable silicosis, and 3 had definite silicosis [46]. Since the publication of Dr. Erasmus’ study, other reports on the occurrence of progressive SSc among those with significant exposure to silica have been published. However, it was not until a decade later that silica exposure was recognized as a predisposing factor to SSc, finally using the eponym “Erasmus syndrome” to describe this condition [47].
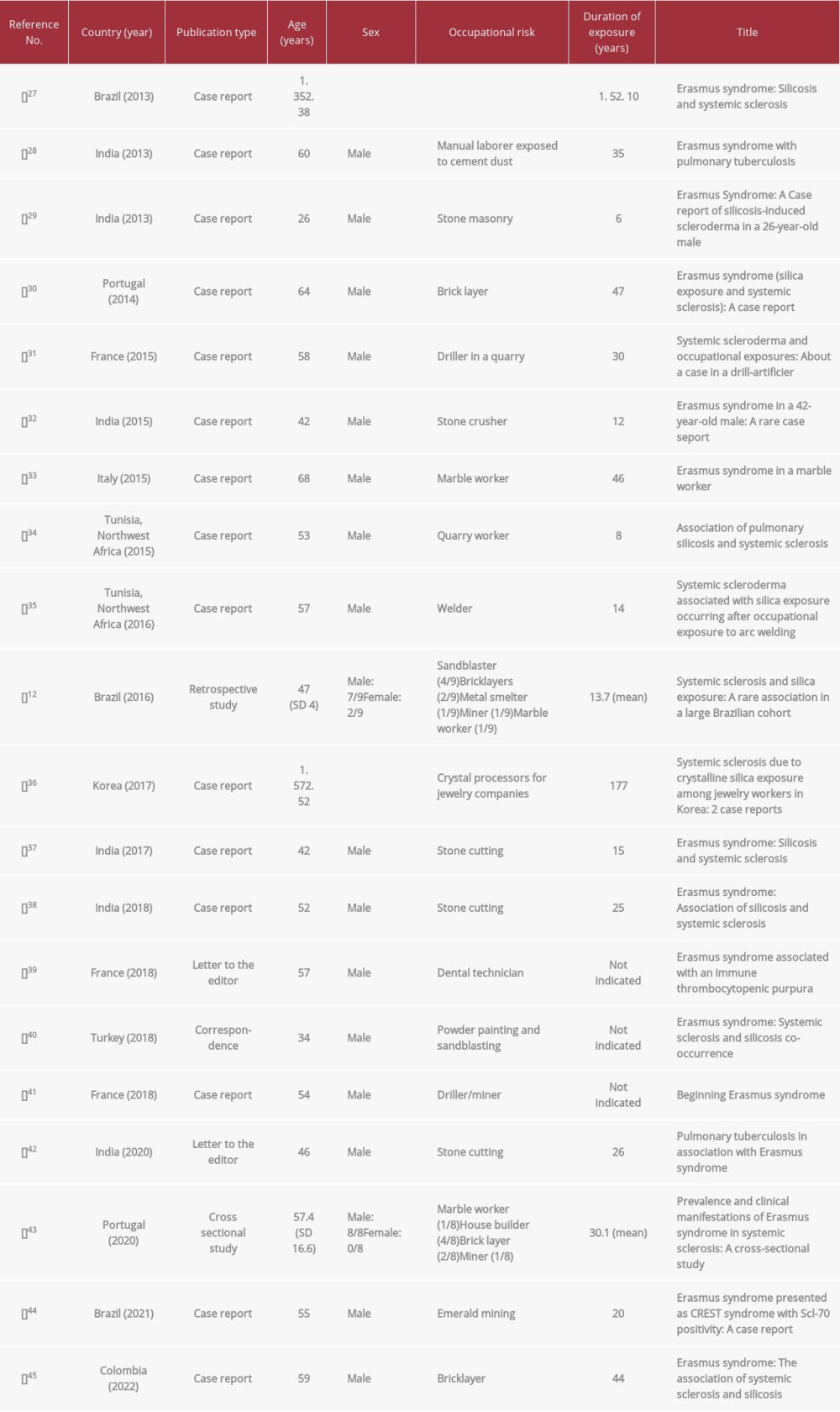
We searched the literature for full-text case reports and reviews on Erasmus syndrome in the past 10 years using the search terms “Erasmus syndrome”, “silica”, and “systemic sclerosis”. Poster presentations and/or abstracts were not included because of the limited information that can be derived from these publication types. We were able to retrieve 20 articles on Erasmus syndrome published between 2012 and 2022. A summary of the retrieved articles is provided in Table 2. Fifteen of these publications were case reports, 1 was a correspondence, another was a retrospective observational study, 2 were editorial letters, and the last was a cross-sectional study. The majority of the patients were male, emphasizing the occupational relevance of Erasmus syndrome; only 2 female patients were reported to have this condition. Regardless of the sex, all of the patients had occupational risk exposure to silica (Table 2). Duration of exposure before developing Erasmus syndrome varied significantly from 5 to 47 years. Most of these patients initially presented with progressive dyspnea prior to developing symptoms related to SSc such as Raynaud’s phenomenon, arthralgia, induration and thickening of fingers, skin tightening and salt-and-pepper dermopathy. Antibodies against Scl-70, the autoantibodies classically associated with SSc, confirmed the presence of this condition among the silica-exposed patients.
The specific pathogenesis involved in the development of SSc after silica exposure remains to be elucidated. Zaghi and colleagues (2010) suggested that silica exposure induces apoptosis and subsequent release of interleukins, TNF-alpha, reactive oxygen species, and products of arachidonic acid metabolism [49]. Overproduction of TNF-alpha, including other fibrogenic factors such as transforming growth factor (TGF)-alpha and epidermal growth factor stimulates uncontrolled collagen synthesis and deposition in various tissues [2]. When alveolar macrophages containing silica die, the silica particles are released and are re-engulfed by other macrophages, perpetuating the inflammatory cycle. Moreover, silica-induced macrophage apoptosis may exacerbate immune-mediated responses by exposing epitopes that are targeted by the body’s immune system [49]. The immune dysregulation brought about by the persistent inflammation is also reinforced by the findings of various antibodies among patients with silica-induced SSc [48]. A study by Lee et al (2017) on silica-induced dysregulation of autoimmunity demonstrated that silica exposure causes antibody production against ANA, Scl-70, anti-centromere protein B, and Sjogren syndrome-related antigen [48].
The gold standard in the management of Erasmus syndrome is avoidance of exposure to silica dust. Treatment of silica-related SSc is no different from that for idiopathic forms of SSc. In general, the approach to the management of SSc is organ-based symptomatic therapy [24,50]. For instance, Raynaud’s phenomenon is alleviated with calcium-channel blockers, those complaining of gastroesophageal reflux are given proton-pump inhibitors, while those with arthralgia and/or myalgia receive short courses of non-steroidal anti-inflammatory drugs [50]. Patients with diffused and progressive cutaneous involvement and/or severe organ involvement are given systemic immunosuppressive therapy such as cyclophosphamide, mycopheno-late mofetil, or methotrexate [50].
Conclusions
Chronic silica exposure is a risk factor for the development of silicosis. The clinical course of patients with silicosis may be complicated by SSc. Maintaining a high index of suspicion is key to the diagnosis of Erasmus syndrome. The present report emphasizes the importance of preventive measures and surveillance among those with occupational exposures to silica. To our knowledge, this is the first documented case of Erasmus syndrome in the Philippines.
Figures
References:
1.. Barnes H, Goh NSL, Leong TL, Hoy R, Silica-associated lung disease: An old-world exposure in modern industries: Respirology, 2019; 24(12); 1165-75
2.. Greenberg MI, Waksman J, Curtis J, Silicosis: A review: Dis Mon, 2007; 53(8); 394-416
3.. Rosenman KD, Reilly MJ, Henneberger PK, Estimating the total number of newly-recognized silicosis cases in the United States: Am J Ind Med, 2003; 44(2); 141-47
4.. Casey ML, Mazurek JM, Silicosis prevalence and incidence among Medicare beneficiaries: Am J Ind Med, 2019; 62(3); 183-91
5.. Pollard KM, Silica, silicosis, and autoimmunity: Front Immunol, 2016; 7; 97
6.. Costantini L, Gilberti R, Knecht D, The phagocytosis and toxicity of amorphous silica: PLoS One, 2011; 6(2); e14647
7.. Leung C, Yu I, Chen W, Silicosis: Lancet, 2012; 379(9830); 2008-18
8.. Parks CG, Conrad K, Cooper GS, Occupational exposure to crystalline silica and autoimmune disease: Environ Health Perspect, 1999; 107(Suppl. 5); 793-802
9.. Mlika M, Adigun R, Bhutta BS, Silicosis. [Updated 2022 Feb 9]: StatPearls [Internet], 2022, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK537341
10.. Miller FW, Alfredsson L, Costenbader KH, Epidemiology of environmental exposures and human autoimmune diseases: Findings from a National Institute of Environmental Health Sciences Expert Panel Workshop: J Autoimmun, 2012; 39(4); 259-71
11.. Shtraichman O, Blanc PD, Ollech JE, Outbreak of autoimmune disease in silicosis linked to artificial stone: Occup Med (Lond), 2015; 65(6); 444-50
12.. Rocha LF, Luppino Assad AP, Marangoni RG, Systemic sclerosis and silica exposure: a rare association in a large Brazilian cohort: Rheumatol Int, 2016; 36(5); 697-702
13.. Qi XM, Luo Y, Song MY, Pneumoconiosis: current status and future prospects: Chin Med J (Engl), 2021; 134(8); 898-907
14.. Go LHT, Cohen RA, Coal workers’ pneumoconiosis and other mining-related lung disease: new manifestations of illness in an age-old occupation: Clin Chest Med, 2020; 41(4); 687-96
15.. Davis GS, Pathogenesis of silicosis: Current concepts and hypotheses: Lung, 1986; 164(3); 139-54
16.. Lanzafame M, Vento S, Mini-review: Silico-tuberculosis: J Clin Tuberc Other Mycobact Dis, 2021; 23; 100218
17.. Ehrlich R, Akugizibwe P, Siegfried N, Rees D, The association between silica exposure, silicosis and tuberculosis: A systematic review and meta-analysis: BMC Public Health, 2021; 21(1); 953
18.. Sharma N, Kundu D, Dhaked S, Das A, Silicosis and silicotuberculosis in India: Bull World Health Organ, 2016; 94(10); 777-78
19.. van den Hoogen F, Khanna D, Fransen J, 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative: Ann Rheum Dis, 2013; 72(11); 1747-55
20.. Sobolewski P, Maślińska M, Wieczorek M, Systemic sclerosis – multidisciplinary disease: clinical features and treatment: Reumatologia, 2019; 57(4); 221-33
21.. Hughes M, Herrick A, Systemic sclerosis: Br J Hosp Med, 2019; 80(9); 530-36
22.. Nikpour M, Stevens WM, Herrick AL, Proudman SM, Epidemiology of systemic sclerosis: Best Pract Res Clin Rheumatol, 2010; 24(6); 857-69
23.. Adigun R, Goyal A, Hariz A: Systemic sclerosis. [online] 2022 [cited 2022 Apr 24]. Available from: Ncbi.nlm.nih.gov
24.. Varga J, Systemic sclerosis (scleroderma) and related disorders: Harrison’s Principles of Internal Medicine, 1998; 2546-60, US, McGraw-Hill Education
25.. Mouthon L, Bérezné A, Bussone G, Scleroderma renal crisis: A rare but severe complication of systemic sclerosis: Clin Rev Allergy Immunol, 2011; 40(2); 84-91
26.. Allanore Y, Simms R, Distler O, Systemic sclerosis: Nat Rev Dis Primers, 2015; 1; 15002
27.. de Miranda AA, Nascimento AC, Peixoto IL, Erasmus syndrome: Silicosis and systemic sclerosis: Rev Bras Reumatol, 2013; 53(3); 310-13
28.. Goyal A, Madan K, Singh N, Erasmus syndrome with pulmonary tuberculosis: BMJ Case Rep, 2013; 2013; bcr2013010443
29.. Ganguly J, Kumar A, Samanta SK, Erasmus syndrome: A case report of silicosis-induced scleroderma in a 26-year-old male: Oman Med J, 2013; 28(5); e058
30.. Silva CS, Silva GT, Andadre L, Saraiva A, Erasmus syndrome (silica exposure and systemic sclerosis): A case report: Galicia Clinic, 2014; 75(1); 33-35
31.. Boulanger M, Bienvenu B, Marquignon MF, [Systemic sclerosis and occupational exposures: About a case in a driller-powderman.]: Rev Med Interne, 2015; 36(8); 551-54 [in Freanch]
32.. Chakrabarti S, Pan K, Erasmus syndrome in a 42-year-old male: A rare case report: J Clin Diagn Res, 2015; 9(5); OD01-3
33.. Bello S, Rinaldi A, Trabucco S, Erasmus syndrome in a marble worker: Reumatismo, 2016; 67(3); 116-22
34.. Ben Abdelghani K, Fazaa A, Souabni L, Zakraoui L, Association of pulmonary silicosis and systemic sclerosis: BMJ Case Rep, 2015; 2015; bcr2013202509
35.. Alaya Z, Kalboussi H, Osman W, Naouar N, [Silica-associated systemic sclerosis occurring after an occupational exposure to arc welding.]: Pan Afr Med J, 2016; 25; 70 [in French]
36.. Kim JY, Do SY, Moon YH, Systemic sclerosis due to crystalline silica exposure among jewelry workers in Korea: two case reports: Ann Occup Environ Med, 2017; 29; 18
37.. Rathore Y, Jain S, Joshi V, Khippal N, Erasmus syndrome: Silicosis and systemic sclerosis: Indian J Occup Environ Med, 2017; 21(2); 94
38.. Sharma R, Sharma A, Sharma A, Erasmus syndrome: Association of silicosis and systemic sclerosis: Indian Dermatol Online J, 2018; 9(3); 185
39.. Fouchard M, Pan Petesch B, Abasq-Thomas C, Erasmus syndrome associated with an immune thrombocytopenic purpura: J Eur Acad Dermatol Venereol, 2018; 32(7); e261-62
40.. Sarı Sürmelİ Z, Oruçoğlu N, Erasmus syndrome: systemic sclerosis and silicosis co-occurrence: Int J Rheum Dis, 2018; 21(6); 1326-29
41.. Ilardo C, Ksiyer S, Louarn A, Marsaudon É, Beginning Erasmus syndrome: Med Therap, 2018; 24(4); 280-82
42.. Sharma RK, Gupta M, Sharma AK, Pulmonary tuberculosis in association with Erasmus syndrome: Indian J Dermatol Venereol Leprol, 2020; 86; 292-95
43.. Azevedo S, Sousa-Neves J, Santos-Faria D, Prevalence and clinical manifestations of Erasmus syndrome in systemic sclerosis: A cross-sectional study: Acta Reumatol Port, 2020; 45(3); 183-90
44.. De Lacerda Pereira F, Portilho J, Erasmus syndrome presented as CREST syndrome with Scl-70 positivity: A case report: Braz J Case Rep, 2021; 1(3); 90-94
45.. Osejo-Betancourt M, Chaparro-Mutiz P, Erasmus syndrome: The association of systemic sclerosis and silicosis: Monaldi Arch Chest Dis, 2022 [Online ahead of print]
46.. Erasmus LD, Scleroderma in gold-miners on the witwatersrand with particular reference to pulmonary manifestations: S Afr J Lab Clin Med, 1957; 3; 209-31
47.. Rodnan GP, Benedek TG, Medsger TA, Cammarata RJ, The association of progressive systemic sclerosis (scleroderma) with coal miners’ pneumoconiosis and other forms of silicosis: Ann Intern Med, 1967; 66; 323-34
48.. Lee S, Hayashi H, Kumaga-Takei N, Autoantibodies in silicosis patients: Silica-induced dysregulation of autoimmunity: Autoantibodies and cytokines [Internet], 2017, London, IntechOpen [cited 2022 May 23]. Available from: https://www.intechopen.com/chapters/58627
49.. Zaghi G, Koga F, Nisihara RM, Autoantibodies in silicosis patients and in silica-exposed individuals: Rheumatol Int, 2010; 30(8); 1071-75
50.. Denton C, Overview of the treatment and prognosis of systemic sclerosis (scleroderma) in adults. UpToDate, 2021 [cited 2022 Apr 24]. Available from:https://www.uptodate.com/contents/overview-of-the-treatment-and-prognosis-of-systemic-sclerosis-scleroderma-in-adults
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250