16 October 2022: Articles 
Physiologic Response to Exercise or Rhabdomyolysis? Creatine Phosphokinase Elevation in 16 Asymptomatic Firefighters
Unusual clinical course, Challenging differential diagnosis
Rajia Arbab12ABCDEF*, Carla Erb12BCDE, Justin Joy2E, Hanady Zainah2EG, Majed Mark Samarneh3BEGDOI: 10.12659/AJCR.937084
Am J Case Rep 2022; 23:e937084
Abstract
BACKGROUND: We present a case series of 16 trainee firefighters who presented to the Emergency Department with elevated creatine phosphokinase levels of greater than 14 000 units per liter 3 days after the initiation of intense aerobic exercise. All 16 patients were diagnosed with exercise-induced rhabdomyolysis and were mostly asymptomatic. While exercise-induced rhabdomyolysis often affects untrained individuals who abruptly initiate strenuous exercises, our patients were all physically well-trained and maintained an active training regimen. In review of this unusual case series, we assess the patients’ risk factors for exercise-induced rhabdomyolysis and the complications of their elevated creatine phosphokinase levels despite their asymptomatic presentations.
CASE REPORT: We focus on the exercise routine, hospital admission, and course of treatment for 4 of the 16 patients who gave written consent to participate in the study. Therapy was targeted towards intravenous fluids and the lowering of creatine phosphokinase levels. Patients 1, 2, 3, and 4 were discharged when creatine phosphokinase levels decreased by 17%, 40%, 39%, and 40%, respectively.
CONCLUSIONS: Given the differing guidelines for diagnosis, treatment, and discharge for asymptomatic exercise-induced rhabdomyolysis, it was unclear if this was a physiologic or pathologic response to exercise, if hospital admission was indicated, and the extent to which creatine phosphokinase had to decrease for discharge. Our aim is to: 1) determine recommendations to prevent muscle injury following exercise, 2) distinguish between physiologic response to exercise and clinically significant muscle damage, and 3) and recommend a course of treatment given asymptomatic presentation.
Keywords: Acute Kidney Injury, rhabdomyolysis, Sports Medicine, creatine kinase, Exercise, Firefighters, Humans, Risk Factors
Background
Rhabdomyolysis is the result of acute skeletal muscle injury and is defined as a clinical syndrome associated with muscle pain and elevation of serum creatine phosphokinase (CPK) with or without myoglobinuria. Associated symptoms of exercise-induced rhabdomyolysis (exRML) are muscle soreness, changes in urine color, nausea, headache, and fatigue [1]. The patho-physiology of skeletal muscle cell death in rhabdomyolysis involves depletion of cellular energy in the form of adenosine triphosphate (ATP). ATP depletion further causes disruption of the plasma membrane, cell death, and release of intracellular muscle components, including CPK, myoglobin, aspartate aminotransferase (AST), alanine transaminase (ALT), aldolase, lac-tate dehydrogenase (LDH), and electrolytes [2].
CPK is the most sensitive indicator of rhabdomyolysis, and normal levels range from 26 to 308 units per liter (U/L) [3]. No absolute CPK elevation is defined for diagnosis of rhabdomyolysis, as CPK levels should be considered in the context of clinical findings. Still, CPK elevations of 5 times the upper limit of normal (ULN) or 1000–1500 U/L are considered suggestive of rhabdomyolysis [4], while other sources suggest 5000 U/L [2].
Timely diagnosis of rhabdomyolysis is essential, as release of these injurious markers can result in complications such as acute kidney injury (AKI), disseminated intravascular coagulation (DIC), and electrolyte abnormalities. AKI is the most significant complication in rhabdomyolysis, as reported frequency ranges from 15% to over 50% of rhabdomyolysis hospitalizations [5]. AKI, in the event of rhabdomyolysis, occurs as a combination of decreased renal perfusion (due to intravascular volume depletion from shifting of fluid into damaged muscle tissue) and direct renal tubular toxicity due to myoglobin.
Myoglobin, a component of skeletal muscle cells, which is released in rhabdomyolysis, is thought to contribute to this tubular cell damage and ischemia through exacerbation of vasoconstriction and direct nephrotoxicity. In the setting of underlying acidemia, myoglobin degrades at pH levels below 5.5 to release ferrihemate [6]. Ferrihemate catalyzes free radical production and ischemic damage, resulting in nephrotoxicity [1]. Myoglobin is a key component of AKI in rhabdomyolysis and results in the dark-colored urine observed in these cases.
While there are many recommendations for the hospitalization and treatment of patients with rhabdomyolysis, consensus criteria for rhabdomyolysis are lacking, particularly for asymptomatic patients. Hospital treatment for rhabdomyolysis focuses on fluid resuscitation until CPK downtrends. While no specific CPK value for discharge is established, several sources recommend intravenous hydration until CPK of 1000 U/L is achieved [7]. In the case of non-traumatic rhabdomyolysis, no significant difference was found in the rate of reduction using normal saline (NS) vs lactated ringers (LR) [8]. The adequate length of hospitalization is also not clearly defined; however, it is suggested that CPK levels are monitored to their peak and when they begin to downtrend, the patient can be safely discharged [9].
Treatment of the acute phase of rhabdomyolysis must focus on preserving renal function, resolving compartment syndrome, restoring metabolic derangements, and volume replacement [10]. To prevent AKI in the case of rhabdomyolysis, aggressive volume resuscitation is indicated to prevent hypovolemia and maintain renal blood flow. Additionally, alkalinization of urine to pH 6.5 has been proposed to reduce the incidence of myoglobin breakdown to toxic metabolites and decrease the risk of damage to tubule cells; however, alkalinization without additional fluid resuscitation has not proven to be effective [11].
While there are many causes of rhabdomyolysis, risk factors of exRML are high or very low temperatures, extreme exercise in unaccustomed individuals, dehydration, creatine supplements, caffeine, and concurrent use of drugs/alcohol [12]. The list of drugs shown to cause or exacerbate rhabdomyolysis is extensive and includes, but is not limited to, statins, daptomycin, colchicine, and succinylcholine. Drugs of abuse, such as alcohol and cocaine, have been shown to cause rhabdomyolysis [13]. Statins have been tightly associated with causing rhabdomyolysis, and this response can be exacerbated by simultaneous use of drugs that inhibit their clearance. Statins, which are HMG-CoA reductase inhibitors, are oxidized by CYP-3A4 in the liver to be cleared. Concurrent use of CYP-3A4 inhibitors, such as macrolides, antifungals, and protease inhibitors, can predispose to rhabdomyolysis [14]. In seeking potential causes of rhabdomyolysis, it is not only important to consider drugs, but also consider drug–drug interactions.
ExRML is a rare phenomenon, and the presentation of 16 fire-fighters at our local firefighter academy with alarmingly high CPK levels is concerning for AKI. This study is of particular interest for asymptomatic athletes in routine training who have not been assessed for CPK levels and might be unaware of their risk of AKI in the event of undiagnosed exRML. In evaluation of this case series, we address possible risk factors that may have resulted in muscle injury, and we suggest recommendations for athletes to prevent exRML and its complications during training. Given the differing guidelines for diagnosis, treatment, and discharge for asymptomatic exRML, we discuss this unusual case series and suggest recommendations for treatment.
Case Reports
FIREFIGHTER ACADEMY EXERCISE ROUTINE AND FIRST RHABDOMYOLYSIS ADMISSION:
Sixteen men in their 20s and 30s with no significant past medical histories presented to the Emergency Department (ED) on the same day in March 2022 after laboratory test results showed elevated CPK levels. Two days prior to admission, the patients had all undergone an intense physical training program at the Fire Department Academy. One of the trainees was admitted to the hospital for a minor knee injury and was found to have elevated CPK levels. Following this discovery, the remaining 15 trainees were tested by Occupational Health, and all 16 fire-fighters in training had CPK >14 000 U/L and were all diagnosed with exRML. Apart from 1 patient who reported a single episode of cola-colored urine, the patients were asymptomatic and denied any pain or discomfort. We focus our report on 4 patients who gave written consent to participate in the study.
On February 28 and March 1, 2022, at 6: 15 a.m., the 16 physically fit men, all with previous athletic and/or military training, reported for days 1 and 2 of their 2 weeks of training at the Fire Department Academy. A day at the academy begins with 1 h of physical training followed by 6 h of classroom time. Temperatures on both days were around −4ºC. Three out of the 4 patients reported having coffee, but none of the patients reported use of any supplements. After entering the academy, they participated in a 1-h exercise routine from 6: 30 a.m. to 7: 30 a.m. without warm-up exercises. The exercise routine included a 2-mile run, a set of 20 push-ups, flutter kicks, leg raises, planks, and climbing up/down 20 flights of stairs 15 times with both hands over their heads. This intense full-body workout was completed without any rests or hydration. The 1-h physical training was followed by immediate return to the classroom, where they completed 6 h of lecture. The patients reported they were accustomed to strenuous exercises of this nature, but the absence of breaks and opportunities to hydrate between sets were unusual compared to previous training experiences.
ADMISSION AND EVALUATION OF PATIENTS:
On admission, the patients were alert and oriented and denied any symptoms of rhabdomyolysis, including pain, nausea, headache, and fatigue. Thorough individual and family medical histories were obtained, and all 4 patients denied medical or surgical history, previous risk factors or symptoms of rhabdomyolysis, previous history of toxic/drug agents, previous episodes of urine color changes, and any family history of genetic disorders or exercise-related muscle strain. Our patients denied use of medical or recreational drugs, including statin use, cannabis, or binge drinking history over the past month.
In obtaining social history, binge drinking was defined as consumption of more than 5 drinks in 1 night.
Patient demographics are presented in Table 1. On physician exams, forearm and thigh muscle massages did not elicit pain in the patients. Electrocardiograms for all patients showed normal sinus rhythm and rate. Toxicology reports, including alcohol screen, from all patients were negative.
Each patient’s CPK level was significantly higher than the ULN as determined by the hospital (26–308 U/L, P<0.05) (Figure 1). Renal function was monitored to determine signs of AKI; however, all 4 patients had baseline creatinine (Cr), blood urea nitrogen (BUN), and electrolyte balance at time of admission and throughout the course of treatment (Table 2). While 1 patient reported a single episode of cola-colored urine prior to hospitalization, urinalysis reports from inpatient and out-patient laboratory tests showed no evidence of blood or proteinuria (Tables 2, 3). AST and ALT enzymes were also elevated (Table 2). While elevated liver enzymes are suggestive of liver injury, they are also intracellular components of muscle and are suggestive of rhabdomyolysis.
Fluid resuscitation with either normal saline (NS) or lactated ringers (LR) was initiated at a rate of 250 mL/h, and CPK levels were monitored (Figure 1). The specific course of treatment and length of stay for each patient is detailed in Table 1. Patients 1, 2, and 4 had urine pH levels of <6.5. Even mildly acidic urine pH levels increase the risk of myoglobin breakdown to toxic metabolites, which can lead to renal tubular injury (Table 2).
PATIENT DISCHARGE AND OUTPATIENT FOLLOW-UP:
The patients’ readiness for discharge was dependent on normal renal function, down-trending CPK levels, and ruling out any life-threatening complications. At the time of discharge, the CPK levels for patients 1, 2, 3, and 4 had decreased by 17% (11,696 U/L), 40% (5600 U/L), 39% (8579 U/L), and 40% (2029 U/L), respectively (Figure 2). Due to elevated CPK levels during discharge, the patients were educated on the importance of aggressive hydration, and follow-up appointments with a nephrologist were made to evaluate kidney function and repeat laboratory tests. The patients were told to return to the ED if they experienced rhabdomyolysis symptoms such as dark urine, muscle pain, or bleeding.
Outpatient laboratory test results 7 days following the initiation of training and 1–4 days after hospital discharge showed CPK levels of 1663 U/L, 548 U/L, 3456 U/L, and 1375 for patients, 1, 2, 3, and 4, respectively (Table 3). While the CPK levels were still elevated, they decreased significantly from CPK levels at discharge by 85.8%, 90.2%, 59.7%, and 32.2%, respectively. BUN, Cr, and electrolytes were within normal ranges for the patients, which suggested normal renal function (Table 3). The CPK values 7 days after any physical exercise and aggressive hydration had markedly decreased. While normal levels were not fully achieved, further intervention was not considered necessary given preserved renal function and down-trending CPK levels.
Discussion
ETIOLOGICAL EVALUATION OF ELEVATED CPK AND ASSESSMENT OF RISK FACTORS:
While the causes of rhabdomyolysis vary, most patients experience only 1 episode of rhabdomyolysis. It is recommended that patients with recurrent cases of rhabdomyolysis seek evaluation for genetic neuromuscular disorders [10]. While glycogen storage diseases were considered in the differential diagnosis, they were considered unlikely given the negative individual and family history of recurrent rhabdomyolysis, exercise intolerance, and neuromuscular disorders. Therefore, they were not evaluated for myophosphorylase deficiency (McArdle’s disease) through genetic testing or muscle biopsy. Outpatient laboratory tests 7 days after training and without any intermittent exercise showed decreased CPK levels averaging 1760.5 U/L (Table 3). While the CPK levels were still above the ULN, the observed decrease from 14 000 U/L following hydration and termination of exercise support that this was a case of exRML rather than underlying myopathy. Additionally, as all 16 fire fighter trainees with elevated CPK levels had undergone intense physical training, exercise-induced rhabdomyolysis was higher on the differential.
We considered other risk factors for rhabdomyolysis, such as drug/alcohol use. The patients denied use of any supplements or medication including statins, history of drug abuse, and all had negative toxicology and blood alcohol concentration (BAC) reports. The 4 patients endorsed histories of social drinking, but they denied binge drinking in the last month. Alcohol has been implicated in up to 20% of episodes of rhabdomyolysis, as the toxin can cause direct injury to the muscle sarcolemma [15]. The patients had elevated AST: ALT ratios of greater than 1.5 at hospital admission, which suggests that alcohol may have contributed to liver and muscle injury (Table 2). Follow-up exams showed moderate elevations of AST and ALT that corresponded closely with the degree of CPK elevation, but the AST: ALT ratios had decreased to closer to 1 (Table 3). While alcohol use was likely not the main culprit in CPK elevation, it is possible that recent alcohol use contributed to muscle damage caused by strenuous exercise through direct injury to sarcolemma as well as inflammation slowing healing [12]. Because the firefighters had been training together, we completed a thorough history of potential environmental causes and exposures that all the firefighters could have been exposed to. None of the firefighters lived together, nor did any of their diets or medical history overlap to cause suspicion. In the absence of other apparent precipitating factors of rhabdomyolysis, such as neuromuscular disorders, significant alcohol/drug abuse, and elevated CPK levels, the patients were assessed and treated for exRML.
PHYSIOLOGIC RESPONSE OR PATHOLOGICAL CAUSE OF CONCERN? PREVENTION OF EXRML:
Proponents of fitness and exercise often claim that some level of muscle injury is necessary for muscle repair and growth. While some studies suggest that exercise-induced muscle damage (EIMD) can promote muscle hypertrophy, this is only in the case of moderate injury and appropriate recovery time [16]. In the case series we observed, the trainees were not given adequate recovery time, as the physical training described was repeated 6 days weekly for 2 weeks, increasing chances of muscle damage. Additionally, the exercises were performed at cold temperatures (−4ºC), which increases the risk of injury [15]. Certain studies suggest that it is customary for athletes to have elevated CPK levels at baseline [17], and the proposed CPK reference interval for male athletes is 82–1083 U/L [18]. While mildly elevated CPK levels in athletes may be a physiologic response to EIMD, the CPK elevations (>14 000 U/L) and elevated AST/ALT levels in this case series are suggestive of concerning muscle damage. When determining whether increases in CPK levels in the case of exRML are physiologic, the extent of the CPK elevation and other markers of muscle injury (eg, AST) must be assessed.
Exercise routines as part of fire department, military, and athletic training should include measures such as warming up, sufficient hydration, moderate temperatures, and breaks for muscle recovery. Hydration during strenuous exercise decreases uric acid concentration, increases urine acidity, reduces the release of toxic components of myoglobin, and reduces the risk of ischemia and vasoconstriction in renal tubules. To allow for muscle recovery, alcohol/drug use should be discouraged, as this can further exacerbate dehydration, the toxic components can cause direct muscle injury, and the inflammation can prevent muscle recovery.
INDICATIONS FOR HOSPITAL ADMISSION AND MARKED CPK ELEVATION IN ASYMPTOMATIC EXRML:
There was a great deal of uncertainty regarding the need for hospitalization given the absence of symptoms of rhabdomyolysis and the patients’ perceived low risk of renal injury suggested by normal BUN and Cr, electrolytes, and negative urinalysis reports (Table 2). Patients 1, 2, and 4 had mildly acidic urine (pH<6.5) which increases the chances of AKI due to myoglobin toxicity in acidic urine environments (Table 2). While CPK levels in this study were elevated to a clinically significant extent, some studies suggest that risk of AKI is low in patients with CPK levels less than 15 000 U/L and is usually precipitated by sepsis, dehydration, or acidosis [19]. Our patients had no significant risk factors for AKI other than dehydration and mild aciduria, but because their CPK levels were concerningly elevated (>14 000), renal injury was still a likely complication.
While there is no strict cut-off for CPK levels, authors of several studies suggest that CPK levels greater than 5 times the ULN or values greater than 1000 U/L are suggestive of rhabdomyolysis [20,21]. The National Lipid Association (NLA) classifies 3 categories of CPK elevation: 1) Mild: less than 10 times the ULN; 2) Moderate: 10–49 times ULN; and 3) Marked: 50 times ULN [22]. The study of Kenney et al suggests that patients with CPK <50 times the ULN, with absence of muscle weakness/swelling, no myoglobinuria, and normal renal function and electrolytes are likely to have physiologic exRML [23]. Our patients had CPK levels >50 times the ULN, which is suggestive of significant skeletal muscle damage and risk of renal injury rather than physiologic exercise-induced elevation of CPK. Therefore, hospital admission was indicated despite lack of apparent symptoms and normal renal function.
HOSPITALIZATION, DECISION TO DISCHARGE, AND OUTPATIENT FOLLOW-UP:
Upon admission, our patients received 3–4 liters of NS or LR at 250 mL/h. There are no concrete guidelines for the desired CPK level for discharge. Some studies recommend achieving CPK levels of 1000 U/L [7] prior to discharge, while other recommendations focus on achieving a urine output of 300 mL/h [4]. Our patients were discharged after they had down-trending CPK levels and repeat laboratory test results suggested preserved renal function. Patients 1, 2, 3, and 4 were discharged when CPK levels decreased by 17% (11 696 U/L), 40% (5600 U/L), 39% (8579 U/L), and 40% (2029 U/L), respectively. The CPK levels of the patients at discharge were starkly contrasting, and it was unclear what CPK level needed to be achieved for safe discharge. Given the minimal risk of complications and preserved renal function, it was unreasonable to prolong hospital stay until CPK levels of 1000 U/L were achieved. Inpatient fluid resuscitation until 1000 U/L would result in unnecessary hospital costs, and the patients were instead recommended to hydrate upon discharge and to have outpatient follow-up with a nephrologist for repeat laboratory tests.
Outpatient laboratory test results 1 week after training and the inciting event for rhabdomyolysis showed that CPK levels had decreased from values at discharge by 85.8% (1663 U/L), 90.2% (548 U/L), 59.7% (3456 U/L), and 32.2% (1375 U/L) for patients, 1, 2, 3, and 4, respectively. The relatively greater improvement in CPK levels following discharge in comparison to inpatient treatment supports our argument that asymptomatic patients who demonstrate a low risk of AKI do not need to be hospitalized until CPK levels completely normalize. Given elevated but down-trending CPK levels in asymptomatic patients, outpatient follow-up with instructions for oral hydration is recommended based on patient compliance and ability to follow up with outpatient care.
NEED FOR GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF RHABDOMYOLYSIS:
Our study emphasizes the need for concrete guidelines for rhabdomyolysis diagnosis, treatment, and discharge. First, the levels of CPK to diagnose rhabdomyolysis with respect to patient demographics (age, athletic ability) must be determined; an elderly patient and an athletic, younger patient require different management with similar CPK levels of 1000 U/L. Second, we must establish methods to distinguish between elevated CPK levels due to rhabdomyolysis and physiologic response to exercise. Lastly, we recommend further research regarding treatment protocol and criteria for discharge considering markers of renal function, risk of complications, and the extent of down-trending CPK levels.
Of note, our study was limited by inconsistent blood draw times and treatment among the patients. The inability of CPK levels to be specific above 14 000 also created some difficulty in both management and treatment of patients due to the lack of a specific baseline. Without an initial value, it became difficult not only to know how aggressively to treat, but also how effective the fluid resuscitation was in managing the CPK levels. We had limited history of years in military training as well as limited work-up for other markers of inflammation to rule out other causes of rhabdomyolysis such as thyroid, metabolic, and neuromuscular disorders. We did not perform biochemical or genetic investigations for neuromuscular disorders such as myophosphorylase deficiency. Further research is needed with a larger dataset to define and establish guidelines for asymptomatic rhabdomyolysis.
Conclusions
In this study, we evaluated 16 asymptomatic firefighters with markedly elevated CPK levels (>14 000) suggestive of exRML and determined their risk factors of strenuous exercise, dehydration, alcohol consumption, and lack of a recovery period.
Their markedly elevated CPK and AST/ALT levels surpassed the reference interval for male athletes, and their presentations suggest pathological cause for concern rather than physiologic response to exercise. Athletic and military training programs should implement scheduled breaks incorporating hydration for muscle recovery and have routine CPK laboratory measurements to distinguish between physiologic EIMD and asymptomatic exRML to prevent complications of exRML, such as AKI.
There is no strict cut-off for CPK elevations in the diagnosis of exRML, but we argue that asymptomatic patients with normal renal function must be evaluated using a higher CPK elevation threshold (eg, >50 times ULN). We propose that admitted patients with asymptomatic exRML with preserved renal function should be discharged when CPK levels downtrend and we recommend outpatient follow-up. Prolonging hospital stay until CPK levels are normalized in asymptomatic exRML leads to unnecessary healthcare costs. Establishing guidelines for diagnosis of rhabdomyolysis and suggestions for hospitalization/ treatment of asymptomatic patients with elevated CPK levels would help decrease healthcare costs and aid in evaluating asymptomatic patients prior to experiencing deadly complications of rhabdomyolysis.
Figures
Tables
Table 1.. Patient summary and their treatment course.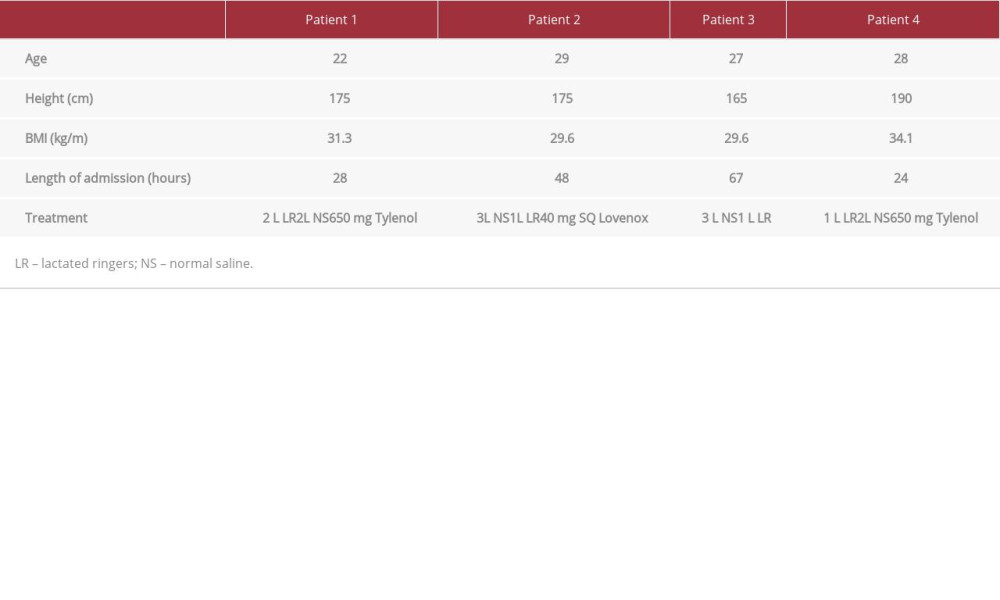 Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels.
Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels.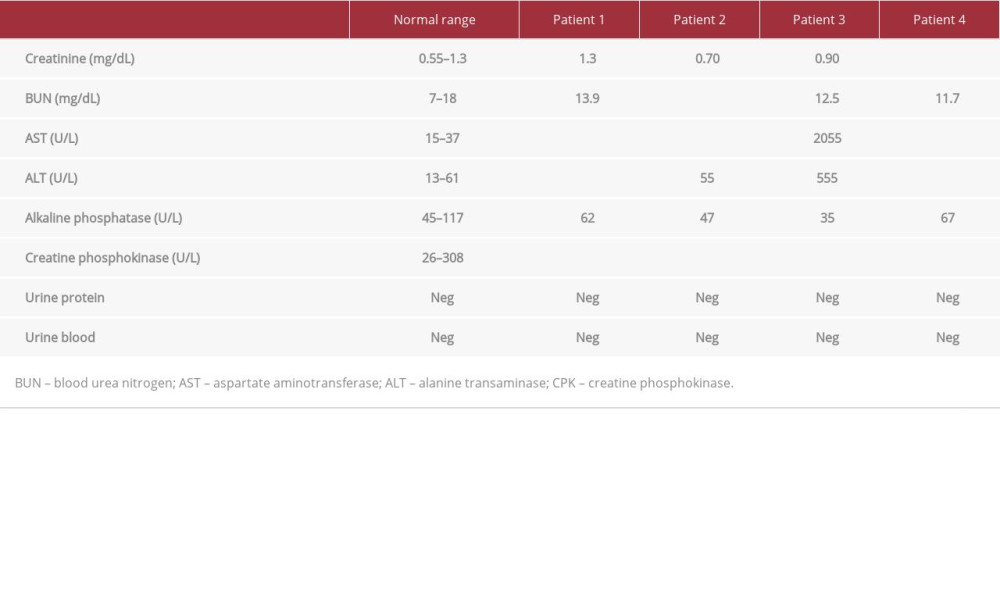 Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded.
Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded.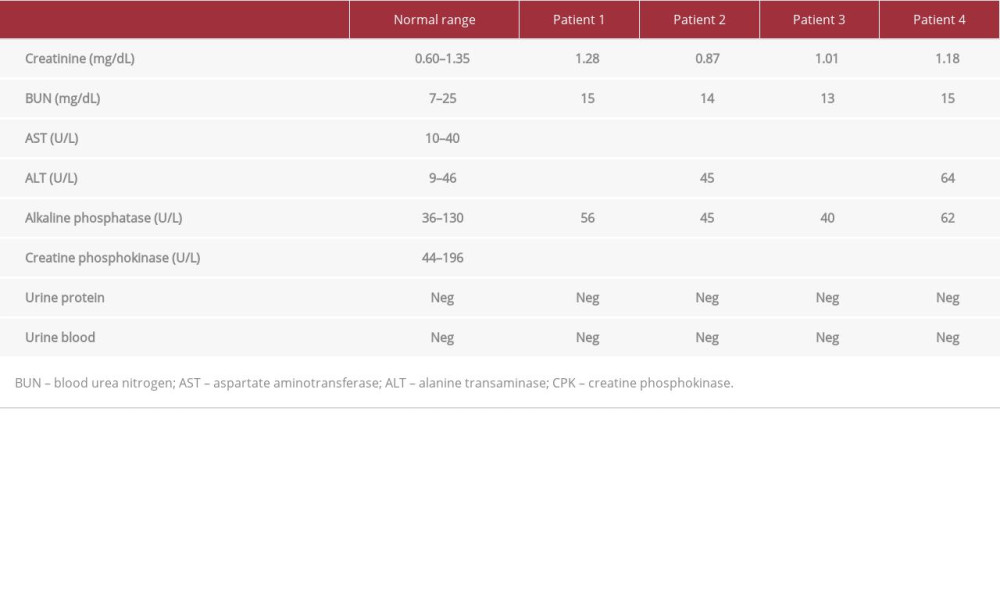
References:
1.. Pearcey GE, Bradbury-Squires DJ, Power KE, Exertional rhabdomyolysis in an acutely detrained athlete/exercise physiology professor: Clin J Sport Med, 2013; 23; 496-98
2.. Al-Ismaili Z, Piccioni M, Zappitelli M, Rhabdomyolysis: Pathogenesis of renal injury and management: Pediatr Nephrol, 2011; 26(10); 1781-88
3.. Criddle LM, Rhabdomyolysis, pathophysiology, recognition and management: Crit Care Nurse, 2003; 23; 14-22
4.. Long B, Koyfman A, Gottlieb M, An evidence-based narrative review of the emergency department evaluation and management of rhabdomyolysis: Am J Emerg Med, 2019; 37(3); 518-23
5.. Melli G, Chaudhry V, Cornblath DR, Rhabdomyolysis: An evaluation of 475 hospitalized patients: Medicine (Baltimore), 2005; 84(6); 377-85
6.. Zager RA, Rhabdomyolysis and myohemoglobinuric acute renal failure: Kidney Int, 1996; 49; 314-26
7.. Eichner ER, Exertional rhabdomyolysis: Curr Sports Med Rep, 2008; 7(1); 3-4
8.. Cho YS, Lim H, Kim SH, Comparison of lactated Ringer’s solution and 0.9% saline in the treatment of rhabdomyosis induced by doxylamine intoxication: Emerg Med J, 2007; 24; 276-80
9.. Ron D, Taitelman U, Michaelson M, Prevention of acute renal failure in traumatic rhabdomyolysis: Arch Intern Med, 1984; 144; 277-80
10.. Zutt R, van der Kooi AJ, Linthorst GE, Rhabdomyolysis: Review of the literature: Neuromuscul Disord, 2014; 24(8); 651-59
11.. Oh RC, Arter JL, Tiglao SM, Larson SL, Exertional rhabdomyolysis: A case series of 30 hospitalized patients: Mil Med, 2015; 180(2); 201-7
12.. Cervellin G, Comelli I, Lippi G, Rhabdomyolysis: Historical background, clinical, diagnostic and therapeutic features: Clin Chem Lab Med, 2010; 48(6); 749-56
13.. Mendes P, Robles PG, Mathur S, Statin-induced rhabdomyolysis: A comprehensive review of case reports: Physiother Can, 2014; 66(2); 124-32
14.. Coco TJ, Klasner AE, Drug-induced rhabdomyolysis: Curr Opin Pediatr, 2004; 16(2); 206-10
15.. Shmouni F, Mcleod K, Sirrs S, Recurrent exercise-induced rhabdomyolysis: CMAJ, 2012; 184(4); 426-30
16.. Schoenfeld BJ, Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy?: J Strength Cond Res, 2012; 26(5); 1441-53
17.. Meyer T, Meister S, Routine blood parameters in elite soccer players: Int J Sports Med, 2011; 32; 875-81
18.. Mougios V, Reference intervals for serum creatine kinase in athletes: Br J Sports Med, 2007; 41(10); 674-78
19.. Bosch X, Poch E, Grau JM, Rhabdomyolysis and acute kidney injury: N Engl J Med, 2009; 361(1); 62-72
20.. Chavez L, Leon M, Einav S, Varon J, Beyond muscle destruction: A systematic review of rhabdomyolysis for clinical practice: Crit Care, 2016; 20; 135
21.. Gabow PA, Kaehny WD, Kelleher SP, The spectrum of rhabdomyolysis: Medicine (Baltimore), 1982; 61; 141-52
22.. Visweswaran P, Guntupalli J, Rhabdomyolysis: Crit Care Clin, 1999; 15; 415-28
23.. Kenney K, Landau ME, Gonzalez RS, Serum creatine kinase after exercise: Drawing the line between physiological response and exertional rhabdomyolysis: Muscle Nerve, 2012; 45(3); 356-62
Figures
Tables
 Table 1.. Patient summary and their treatment course.
Table 1.. Patient summary and their treatment course. Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels.
Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels. Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded.
Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded. Table 1.. Patient summary and their treatment course.
Table 1.. Patient summary and their treatment course. Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels.
Table 2.. Laboratory test values upon admission. Abnormal values are bolded and include the elevated CPK levels for all 4 patients in addition to elevated AST and ALT levels. Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded.
Table 3.. Laboratory values on follow-up. Patient 1 was tested 4 days after discharge, patient 2 was tested 3 days after discharge, and patients 3 and 4 were tested 1 day after discharge. All 4 patients’ CPK levels were still above the normal range, but the values showed a downtrend since discharge. Abnormal values are bolded. In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








