27 December 2022: Articles 
A 14-Year-Old Saudi Boy with Gynecomastia, Cushing Syndrome, Large-Cell Calcifying Sertoli Cell Tumor of the Testis, and Carney Complex
Challenging differential diagnosis, Rare disease
Tahrir Khalf Alruwaili1ABDEF, Khalid Ibrahim Alkanhal1ABDEF, Anas M. AlShoomiDOI: 10.12659/AJCR.937404
Am J Case Rep 2022; 23:e937404
Abstract
BACKGROUND: Carney complex (CNC) is a rare multiple neoplasia syndrome with autosomal dominant inheritance. CNC is frequently misdiagnosed owing to its diverse clinical characteristics. We reported the case of a 14-year-old Saudi boy with a history of gynecomastia, Cushing syndrome, large-cell calcifying Sertoli cell tumor of the testis, and CNC.
CASE REPORT: The patient was referred to the pediatric endocrine clinic for evaluation of bilateral slow progressing gynecomastia for 1-year duration. His clinical examination revealed lentigenes, bilateral diffuse breast enlargement (consistent with Tanner stage III), and asymmetrical testicular enlargement, more on the left side. Other systemic examinations were unremarkable. The initial blood workup showed elevated estradiol level with unsuppressed cortisol after an overnight 1-mg dexamethasone suppression test. Breast ultrasound (US) confirmed true gynecomastia. Testicular US revealed microcalcification and the testicular biopsy confirmed diagnoses of large-cell calcifying Sertoli cell tumor (LCCSCT). A 2-step dexamethasone suppression test showed a paradoxical rise in serum and urine cortisol levels, which are characteristic for PPNAD. LCCSCT and PPNAD are 2 major criteria fulfilling a diagnosis of CNC. The gene test showed heterozygous mutation in the PRKAR1A gene, which is diagnostic for CNC. The patient underwent bilateral mastoplasty and was planned for radical left orchiectomy.
CONCLUSIONS: Gynecomastia and LCCSCT can be presenting features of CNC, which mandates careful, thorough clinical examination and tailored investigation to reach a diagnosis.
Keywords: carney complex, Sertoli Cell Tumor, Male, Child, Humans, Adolescent, Cushing Syndrome, gynecomastia, Hydrocortisone, Saudi Arabia, Sertoli Cells, Testicular Neoplasms, Dexamethasone
Background
Carney complex (CNC) is a rare genetic disorder involving multiple neoplasia syndrome, first described in 1985 [1]. More than 50% of cases are familial owing to an autosomal dominant inheritance pattern, or appear occasionally as a result of a de novo genetic defect [2].
The pathogenesis of PPNAD is unclear, but it appears to be associated with pathogenic variants of the
Sertoli cell tumor is a rare subtype of sex cord-stromal tumors and it contributes to less than 1% of all testicular tumors [12,13]. However, there are 3 subtypes of Sertoli cell tumors: not otherwise specified, and 2 variants (sclerosing Sertoli cell tumors and LCCSCTs) [13]. LCCSCT is an uncommon variant of Sertoli cell tumor [14]. LCCSCT is typically described by distinguishing histology: an intratubular growth pattern, with hyalinization of the tubular basement membrane and cells arranged in circumscribed nodules. Large round Sertoli cells with abundant cytoplasm are characteristic, in addition to massive calcifications, which are essential for the diagnosis of LCCSCT [14]. To the best of our knowledge, our case report is the first to describe LCCSCT in a Saudi boy associated with CNC for which conservative treatment was initially provided along with regular follow-up. Conservative treatment is initially indicated if there is no sign of distressing malignancy. Physical and ultra-sonographic examinations are necessary once a year as part of the follow-up [15]. Aromatase inhibitors can be an efficacious treatment option for patients with LCCSCT [16], but data from clinical trials are needed. The present case is comparable to that of an adolescent who presented with a testicular mass at age 9 years followed by PPNAD diagnosed at age 22 years [1].
CNC is a rare condition, but over 750 patients have been described in different ethnic groups [7]. Given the significant variability in the clinical manifestations, even within a given family, careful clinical evaluation of first-degree family members is warranted in presumed “sporadic” cases [17]. Familial transmission has been reported more frequently via the affected mother, suggesting a non-Mendelian inheritance or impaired male fertility of affected individuals [17]. Early diagnosis allows vigilant continuing observation of tumors and complications, thus improving disease prognosis by early treatment. Herein, we report the case of a 14-year-old boy who had CNC with large-cell calcifying Sertoli cell tumor (LCCSCT) of the testis and primary pigmented nodular adrenocortical disease (PPNAD).
Case Report
A 14-year-old boy was referred to our Endocrinology Department at King Fahd Medical City (KFMC) in 2017 for evaluation of bilateral breast enlargement and hyperprolactinemia. The bilateral breast enlargement was noticed by the family 1 year prior to presentation. It was slowly progressive but not painful, with no discharge from nipples or galactorrhea, and no skin changes over the breast area. There was no report of headache, vomiting, visual impairment symptoms, or acne. He had no history of intracranial tumor or head irradiation. The parents of the patient were second-degree cousins. The patient was the eldest child among 3 healthy siblings and was developmentally normal, with average school performance.
The examination revealed a healthy-appearing patient who was not dysmorphic, pale, or jaundiced. His weight was 46 kg (90th percentile), height was 156 cm (90th percentile), and body mass index was 18.5 kg/m2 (80th percentile). Lentigines were found on the membrane lining the eyes and on the sclera (Figure 1). There was hyperpigmentation of the gingiva. Bilateral diffuse breast enlargement was consistent with Tanner stage III. Also, he had neither focal breast lesion nor palpable axillary lymph nodes. Pubic Hair Tanner stage was II. The physical examination also revealed enlargement of the left testis.
The clinical laboratory findings revealed a serum prolactin level of 576 ng/mL (normal is less than 20 ng/ml) and serum estradiol 178 pg/mL (normal is 10–50 pg/ml). However, other laboratory findings, such as complete blood count, liver function test, and kidney function test, were normal. The serum sodium and potassium levels were 136 mEq/L and 3.9 mEq/L, respectively. Lactic acid dehydrogenase, luteinizing hormone, follicle-stimulating hormone, testosterone, and dehydroepiandrosterone sulfate were in normal ranges. The β-Human chorionic gonadotropin hormone and α-fetoprotein serum levels showed a normal profile.
An ultrasonography evaluation of the breast revealed prominent fibro-fatty tissue bilaterally, likely a manifestation of physiological gynecomastia. However, there was no evidence of suspicious solid or cystic mass lesions. An ultrasonography evaluation of the testis revealed multiple microcalcifications (Figure 2). The computed tomography (CT) scan of the pelvis revealed bilateral testicular calcification, larger on the left side (Figure 3).
Based on findings of ultrasonography, testicular biopsy was performed, which revealed a large-cell calcifying Sertoli cell tumor (Figure 4). The case was discussed in the Tumor Board meeting and conservative treatment with regular follow-up was initiated.
High-dose adrenocorticotropic hormone (ACTH) stimulation testing revealed a normal profile and did not suggest a diagnosis of adrenal insufficiency. Similarly, molecular genetics report
Molecular genetic analysis (Table 2).
A blood sample was sent to Centogene Lab (Germany), at which a gene panel for LCCSCT using next-generation sequencing (NGS) was done and showed heterozygous, nonsense mutation in the PRKAR1A gene, likely a pathogenic variant, consistent with the genetic diagnoses of Carney complex type 1.
Regarding other possible comorbidities of CNC, a wide examination was performed to exclude the involvement of other organs. Ultrasonography for the thyroid and pelvis, as well as echocardiography, did not reveal abnormalities. Brain and pituitary gland MRI were normal.
Invasive bilateral adrenal venous sampling was done (Table 3), and showed almost double the normal amount of cortisol secretion from the left adrenal gland, which confirmed the presence of left adrenal lateralization (predominance) as a source of cortisol secretion. After confirming the diagnosis, the family members were offered genetic testing for the same mutation (target gene mutation test), the mother and siblings have not carried the mutation (wild-type gene). His father was unable to undergo genetic testing because of special social circumstances. The patient underwent bilateral mastoplasty because of distressing gynecomastia. For the Cushing syndrome, the conservative management vs laparoscopic adrenalectomy was initially discussed with the family, and as the patient was not showing any apparent clinical manifestation of Cushing syndrome (eg, hypertension or abnormal BMI), we agreed on conservative management with regular follow-up.
The patient is 16 years old at present. During regular follow-up visits in the urology clinic, he has been reporting left testicular pain, for which testicular US was repeated and showed significant progression of left testicular calcification, and a radical left orchiectomy was planned. In the endocrine clinic, he started to show intermittent elevation in blood pressure, higher BMI (29–31) kg/m2, and new appearance of pink stria on the abdominal wall. His repeated Hba1c, thyroid function test, and IGF-1 levels were normal. For the Cushing features, 24-h urine collection for urinary free cortisol (UFC) excretion showed a level of 34.7 mcg/m2/day (normal level is less than 70 mcg/m2/day), with follow-up for reevaluation.
Discussion
This case report shows that gynecomastia can be the presenting feature of an underlying multisystem disorder, which mandates thorough examination and tailored investigation to reach a diagnosis. We presented a patient with classic clinical characteristics of CNC, including spotty skin pigmentation on the membrane lining the eyes and on the sclera, LCCSCT, and ACTH-independent Cushing syndrome – PPNAD. The molecular genetic analysis identified a known pathogenic mutation of
The patient has also manifested primary pigmented nodular adrenal disease (PPNAD), in association with ACTH-independent Cushing syndrome (CS). The diagnosis of ACTH-independent CS related to PPNAD can be challenging and the best screening test for CS is measurement of urine free cortisol excretion [18]. The current case report found more than 1000 nmol/L free cortisol in a 24-h urine sample on day 6 of the dexamethasone suppression test. Moreover, a low serum ACTH level of 0.8 (normal range: 5–50 pg/mL) and serum cortisol level of 503 nmol/L were noted. Our patient had a less aggressive course of the CNC in comparison to a previous case report by Rosenblum et al in 2017 [1], in which the patient had LCCSCT, pancreatic cancer, and Cushing syndrome due to adrenocortical carcinoma.
The CT examination of the adrenal gland with PPNAD is often interpreted as normal and occasionally it represents the appearance of a string of beads due to the presence of multiple small nodules in the otherwise atrophic adrenal cortex [19]. However, it is worth emphasizing that, in cases like this, because of the patient’s low levels of ACTH and normal adrenal imaging, a hypothesis of exogenous glucocorticoid must also be persistently investigated and ruled out. Furthermore, it is important to make sure that the sampling and assaying of ACTH have been performed adequately; otherwise, the ACTH values might be falsely found to be low, thus inducing diagnostic error [20]. PPNAD is a rare form of ACTH-independent CS that can appear alone, but 90% of cases are linked to CNC [21]. The symptoms are often mild, and patients typically present long after the onset of symptoms. The disease commonly appears during the second decade of life, but an age range of 4–44 years has been recorded in the literature [17,22]. CS in PPNAD can present as overt CS, subclinical CS, cyclic CS, or atypical (asthenic) CS, of which overt CS is the most common [23]. Our patient was classified as having subclinical CS. The preferred treatment option for PPNAD with overt CS is bilateral total adrenalectomy [24]. A laparoscopic approach has a lower rate of morbidity in comparison to the open technique, and it involves less postoperative pain, shorter length of hospital stay, and low overall cost [19]. In case of surgical failure or before adrenalectomy, pharmacotherapy with ketoconazole, metyrapone, mitotane, and trilostane alone or in combination can control hypercortisolism by inhibiting steroidogenesis. Fluconazole has recently been suggested as a safer alternative to ketoconazole [25].
Conclusions
We presented a case of CNC with gynecomastia, large-cell calcifying Sertoli cell tumor (LCCSCT) of the testis and primary pigmented nodular adrenocortical disease. PPNAD is a rare cause of ACTH-independent CS in childhood and can indicate underlying CNC. Moreover, LCCSCTs are very rare tumors and most of them are benign. It is highly recommended that clinical assessments and genetic tests be conducted promptly in such patients. The account of clinical manifestations of this patient adds to the knowledge about CNC and
Figures
References:
1.. Rosenblum F, Koenig RG, Mikhail FM, An adolescent with large cell calcifying Sertoli cell tumor of the testis and undiagnosed Carney Complex: A case report: Diagn Cytopathol, 2017; 45(7); 634-39
2.. Sandrini F, Stratakis C, Clinical and molecular genetics of Carney complex: Mol genet Metab, 2003; 78(2); 83-92
3.. Bertherat J, Carney complex (CNC): Orphanet J Rare Dis, 2006; 1(1); 21
4.. Papanastasiou L, Fountoulakis S, Voulgaris N, Identification of a novel mutation of the PRKAR1A gene in a patient with Carney complex with significant osteoporosis and recurrent fractures: Hormones, 2016; 15(1); 129-35
5.. Kirschner LS, Carney JA, Pack SD, Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex: Nat Genet, 2000; 26(1); 89-92
6.. Matyakhina L, Pack S, Kirschner LS, Chromosome 2 (2p16) abnormalities in Carney complex tumours: J Med Genet, 2003; 40(4); 268-77
7.. Correa R, Salpea P, Stratakis CA, Carney complex: An update: Eur J Endocrinol, 2015; 173(4); M85-97
8.. Liu Q, Tong D, Liu G, Carney complex with PRKAR1A gene mutation: A case report and literature review: Medicine (Baltimore), 2017; 96(50); e8999
9.. Espiard S, Bertherat J, Carney complex: Front Hom Res, 2013; 41; 50-62
10.. Bertherat J, Horvath A, Groussin L, Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): Phenotype analysis in 353 patients and 80 different genotypes: J Clin Endocrinol Metab, 2009; 94(6); 2085-91
11.. Boikos Sosipatros A, Stratakis Constantine A, Carney complex: The first 20 years: Curr Opin Oncol, 2007; 19(1); 24-29
12.. Sato K, Ueda Y, Sakurai A, Large cell calcifying Sertoli cell tumor of the testis: Comparative immunohistochemical study with Leydig cell tumor: Pathol Int, 2005; 55(6); 366-71
13.. Moch H, Cubilla AL, Humphrey PA, The 2016 WHO classification of tumours of the urinary system and male genital organs – part A: Renal, penile, and testicular tumours: Eur Urol, 2016; 70(1); 93-105
14.. Proppe KH, Scully RE, Large-cell calcifying Sertoli cell tumor of the testis: Am J Clin Pathol, 1980; 74(5); 607-19
15.. Gourgari E, Saloustros E, Stratakis CA, Large-cell calcifying Sertoli cell tumors of the testes in pediatrics: Curr Opin Pediatr, 2012; 24(4); 518-22
16.. Grandone A, del Giudice EM, Cirillo G, Prepubertal gynecomastia in two monozygotic twins with Peutz-Jeghers syndrome: Two years’ treatment with anastrozole and genetic study: Horm Res Paediatr, 2011; 75(5); 374-79
17.. Stratakis CA, Kirschner LS, Carney JA, Clinical and molecular features of the Carney complex: Diagnostic criteria and recommendations for patient evaluation: J Clin Endocrinol Metabol, 2001; 86(9); 4041-46
18.. Navarro Moreno C, Delestienne A, Marbaix E, Familial forms of Cushing syndrome in primary pigmented nodular adrenocortical disease presenting with short stature and insidious symptoms: A clinical series: Horm Res Paediatr, 2018; 89(6); 423-33
19.. Bavadiya G, Roy A, Sarkar KK, Primary pigmented nodular adrenocortical disease (PPNAD) presenting as Cushing syndrome in a child and review of literature: Acta Endocrinol (Buchar), 2020; 16(3); 362-65
20.. Castro M, Moreira AC, Diagnóstico laboratorial da síndrome de Cushing: Arquivos Brasileiros de Endocrinologia & Metabologia, 2002; 46(1); 97-105 [in Portuguese]
21.. Sandrini F, Stratakis C, Clinical and molecular genetics of primary pigmented nodular adrenocortical disease: Arq Bras Endocrinol Metab, 2004; 48(5); 637-41
22.. Manipadam MT, Abraham R, Sen S, Simon A, Primary pigmented nodular adrenocortical disease: J Indian Assoc Pediatr Surg, 2011; 16(4); 160-62
23.. Memon SS, Thakkar K, Patil V, Primary pigmented nodular adrenocortical disease (PPNAD): single centre experience: J Pediatr Endocrinol Metab, 2019; 32(4); 391-97
24.. Powell AC, Stratakis CA, Patronas NJ, Operative management of Cushing syndrome secondary to micronodular adrenal hyperplasia: Surgery, 2008; 143(6); 750-58
25.. Riedl M, Maier C, Zettinig G, Long term control of hypercortisolism with fluconazole: case report and in vitro studies: Eur J Endocrinol, 2006; 154(4); 519-24
Figures
Tables
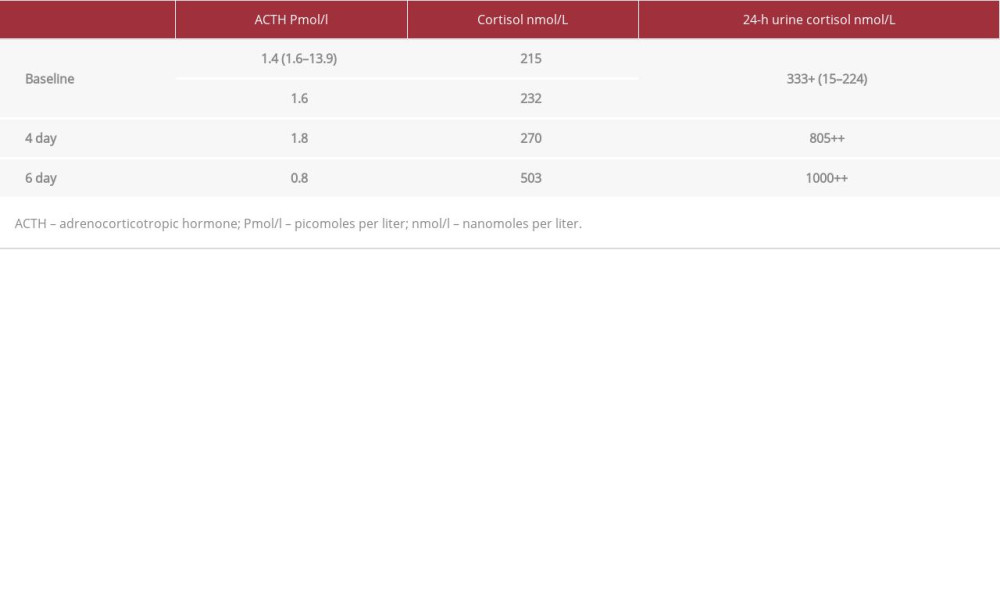 Table 1.. Six-day standard (2-step) dexamethasone suppression test.
Table 1.. Six-day standard (2-step) dexamethasone suppression test.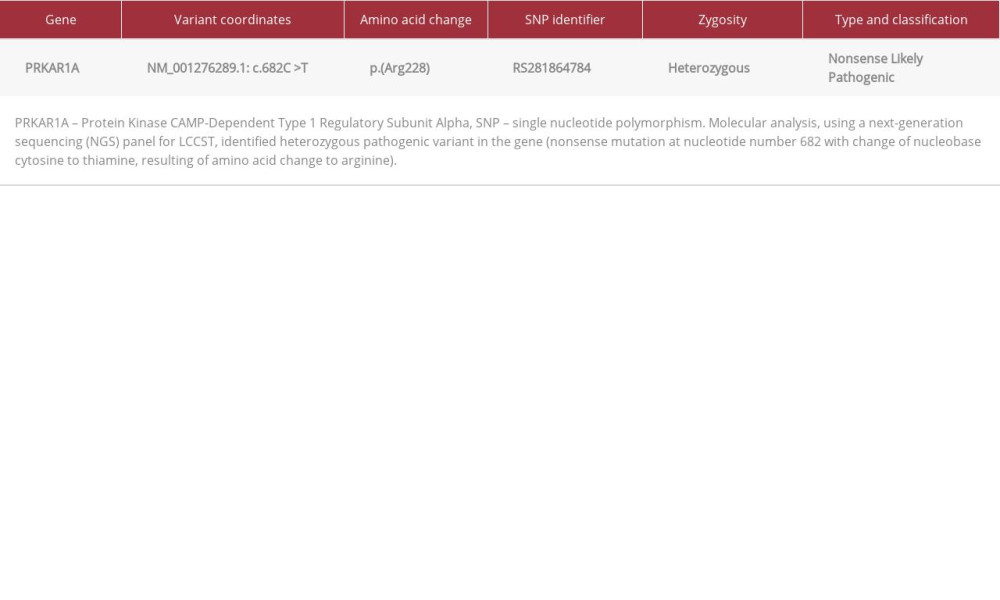 Table 2.. Gene finding description.
Table 2.. Gene finding description.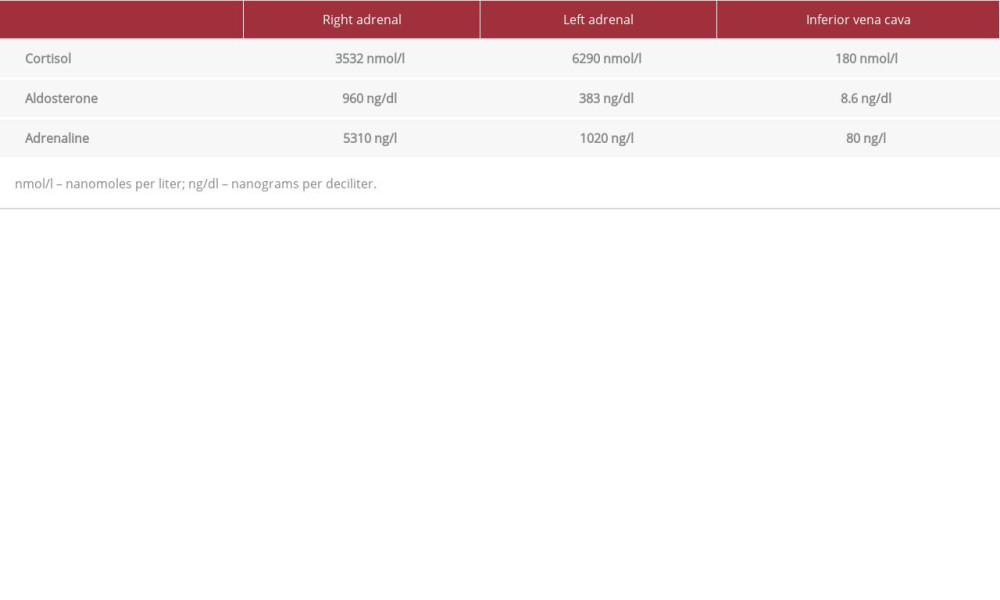 Table 3.. Invasive adrenal venous sampling for cortisol, aldosterone, and adrenaline levels.
Table 3.. Invasive adrenal venous sampling for cortisol, aldosterone, and adrenaline levels. Table 4.. Diagnostic criteria of Carney complex.
Table 4.. Diagnostic criteria of Carney complex. Table 1.. Six-day standard (2-step) dexamethasone suppression test.
Table 1.. Six-day standard (2-step) dexamethasone suppression test. Table 2.. Gene finding description.
Table 2.. Gene finding description. Table 3.. Invasive adrenal venous sampling for cortisol, aldosterone, and adrenaline levels.
Table 3.. Invasive adrenal venous sampling for cortisol, aldosterone, and adrenaline levels. Table 4.. Diagnostic criteria of Carney complex.
Table 4.. Diagnostic criteria of Carney complex. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








