31 December 2022: Articles 
A 75-Year-Old Woman with a 5-Year History of Controlled Type 2 Diabetes Mellitus Presenting with Polydipsia and Polyuria and a Diagnosis of Central Diabetes Insipidus
Rare coexistence of disease or pathology
Nobumasa Ohara1ABCDEF*, Toshinori Takada2D, Yasuhiro Seki3D, Katsuhiko Akiyama3D, Yuichiro YoneokaDOI: 10.12659/AJCR.938482
Am J Case Rep 2022; 23:e938482
Abstract
BACKGROUND: Central diabetes insipidus (CDI) is a rare disorder characterized by large volumes of dilute urine because of a lack of antidiuretic hormone. Co-existing CDI and diabetes mellitus without inherited disorders such as Wolfram syndrome are rare. It is both important and challenging to diagnose this combination because the 2 conditions present with thirst, polydipsia, and polyuria. A few cases of CDI developing in patients with type 2 diabetes mellitus (T2D) have been reported. We report an unusual case of CDI that developed in an older patient with T2D. The aims of this report are to share the clinical course and discuss clues to the early diagnosis of CDI in T2D.
CASE REPORT: A 70-year-old Japanese woman developed T2D with hyperglycemia symptoms, including thirst, polydipsia, and polyuria. After starting medical treatment, the hyperglycemia and its symptoms improved. The glycated hemoglobin level decreased from 9% to 6%. However, 5 years later (at 75 years of age), she re-exhibited thirst, polydipsia, and polyuria despite stable glycemic control. Her urine volume was large (6.3 L/day). A urine glucose test was negative. The plasma osmolality was high (321 mOsm/kg), while the urinary osmolality was low (125 mOsm/kg). A significant increase in urinary osmolality following vasopressin administration indicated a diagnosis of CDI. Desmopressin therapy effectively relieved the symptoms.
CONCLUSIONS: This case highlights the need to consider CDI as a rare but important comorbid disorder in patients with diabetes mellitus, including T2D, particularly those presenting with thirst, polydipsia, and polyuria despite well-controlled glycemia.
Keywords: desmopressin, Central Diabetes Insipidus, Polyuria, Type 2 diabetes mellitus, Female, Humans, Aged, Diabetes Insipidus, Neurogenic, Polyuria, Diabetes Mellitus, Type 2, polydipsia, Hyperglycemia
Background
Central diabetes insipidus (CDI) is a rare endocrine disorder characterized by a large volume of dilute urine because of a lack of the posterior pituitary hormone arginine vasopressin (AVP), also known as antidiuretic hormone [1–3]. Treatment goals for CDI are correcting and stabilizing the water deficit and electrolyte balance, together with reducing the excessive urination and thirst. The synthetic AVP analogue desmopressin is the treatment of choice.
Diabetes mellitus is a metabolic disease characterized by chronic hyperglycemia due to insufficient insulin action [4,5]. Diabetes mellitus can be classified based on the underlying etiology. Type 2 diabetes mellitus (T2D) is the most common form, characterized by a relative (rather than absolute) insulin deficiency and decreased insulin sensitivity.
It is both important and challenging to diagnose co-existing CDI and diabetes mellitus because the 2 conditions present with similar symptoms including thirst, polydipsia, and polyuria [1–4].
There are many reported cases of co-existing CDI and diabetes mellitus in patients with Wolfram syndrome, a rare autosomal recessive neurodegenerative disorder characterized by CDI, juvenile-onset diabetes mellitus, optic nerve atrophy, and sensori-neural deafness [6]. The co-existence of CDI and diabetes mellitus without inherited disorders [6,7] is rare. Co-existing CDI and type 1 diabetes mellitus have been reported in patients with polyglandular autoimmune syndrome [8]. Although the etiological links remain unclear, several cases of CDI with T2D [9–14] or diabetes mellitus of other etiologies [15–18] have been reported.
Although it is important to know the clinical characteristics of CDI that develops in patients being treated for T2D, as the number of patients with T2D has been increasing [19], only a few cases have been reported [10,11]. Here, we report the unusual case of an older patient with T2D who developed CDI. The aims of this report are to share the clinical course and discuss clues to the early diagnosis of CDI in T2D.
Case Report
A 70-year-old Japanese woman visited a primary care doctor due to a 2-month history of thirst, polydipsia, pollakisuria, and polyuria. Her family history included paternal T2D. No family member suffered from deafness, visual loss, diabetes insipidus, or any renal tract or neurological abnormality. The patient had never smoked cigarettes and did not drink alcohol. She had no history of a brain tumor, cerebrovascular disease, brain surgery, or radiation therapy. Her body weight had been approximately 46 kg in her 20s but gradually increased to 51 kg when she was 70 years old; she then developed thirst, polydipsia, pollakisuria, and polyuria. Two months later, she visited a primary care doctor, who noted a 3-kg body weight loss and hyperglycemia with a high glycated hemoglobin (HbA1c) level (9.0%). The patient was diagnosed with T2D accompanied by mild dyslipidemia and hypertension and was started on diet therapy, oral hypoglycemic agents [sitagliptin (50 mg/ day) and metformin (500 mg/day)], and the antihypertensive agent amlodipine (2.5 mg/day). Her hyperglycemia improved gradually, and the thirst, polydipsia, pollakisuria, and polyuria resolved. Her HbA1c level decreased to around 6%.
The patient subsequently developed thirst, polydipsia, pollakisuria, and polyuria at 75 years of age, without any prodrome such as headache or head trauma, and she visited her primary doctor. A blood test revealed fasting plasma glucose and HbA1c levels of 105 mg/dL and 6.7%, respectively; a urine glucose test was negative. She was first thought to have a bladder problem, and oral solifenacin succinate (5 mg/day) was commenced. However, her symptoms persisted, and she was referred to our hospital and admitted.
On physical examination, she was 150 cm tall, weighed 43 kg (body mass index, 19.1 kg/m2), and her body temperature was 36.7°C, blood pressure 140/63 mmHg, and pulse rate 69 beats per minute. Her daily urine volume was large (6.3 L/day). Her mouth was dry. No heart murmurs, chest rales, or peripheral edema were detected.
Laboratory tests (Table 1) revealed high serum sodium (158 mEq/L) and chloride (121 mEq/L) levels, high plasma osmolality (Posm; 321 mOsm/kg), and low urinary osmolality (Uosm; 125 mOsm/kg). The plasma AVP level was relatively low (0.6 pg/mL) [20]. These results suggested hypertonic dehydration and hypotonic polyuria because of inability of the kidneys to conserve body water and concentrate urine. We considered diabetes insipidus.
Because the patient had severe plasma hyperosmolality, we did not perform the water deprivation test or hypertonic saline test [2,3,21]. Instead, we performed the vasopressin administration test, which revealed a drastic rise in Uosm from 73 to 509 mOsm/kg, along with a reduced urine volume from 140 to 15 mL/30 min, within 2 hours after intravenous vasopressin (5 units) administration (Table 2). The significant increase in Uosm by up to 100% ruled out nephrogenic diabetes insipidus and indicated a diagnosis of CDI [2,3,21].
Magnetic resonance imaging (MRI) detected no morphological abnormalities in the brain including the hypothalamus, hypophyseal stalk, or pituitary gland. However, the high signal intensity of the posterior pituitary on T1-weighted images was absent. These results were consistent with idiopathic CDI [2,3,22].
The patient was started on oral desmopressin at 60 μg/day to treat CDI on day 4 of admission. The dose was titrated up gradually on the basis of her polyuria, thirst, and electrolyte imbalance.
Dynamic tests for anterior pituitary hormone secretion revealed no anterior pituitary dysfunction.
To investigate possible co-existing autoimmune disorders [23], serum autoantibodies against different organs were examined, and the patient tested positive for thyroglobulin autoantibody (94.0 IU/mL; reference range, <28.0 IU/mL). Neck ultrasonography revealed coarse, low echogenicity in a normal-sized thyroid gland, suggesting Hashimoto thyroiditis [24]. Because the patient had normal serum thyroid-stimulating hormone (3.79 μIU/mL) and free thyroxine (1.11 ng/dL) levels (Table 1), thyroid hormone replacement therapy was not indicated.
Regarding the patient’s diabetes mellitus, her fasting plasma glucose was 114 mg/dL and HbA1c level 6.6% (Table 1). Her serum immunoreactive insulin level was 3.4 µU/mL and C-peptide immunoreactivity 1.8 ng/mL. Tests for pancreatic islet autoantibodies, such as glutamic acid decarboxylase autoantibody, insulinoma-associated antigen-2 antibody, insulin antibody, islet cell antibody, and zinc transporter 8 antibody, were negative. These findings were consistent with a diagnosis of T2D with a relatively preserved endogenous insulin secretion capacity. Funduscopy detected no diabetic retinopathy. The patient had no numbness in her hands or feet and had normal Achilles tendon reflexes.
Upon treatment of the CDI, after the desmopressin was increased to 300 μg/day, the urine volume decreased to around 1 L/day (Figure 1). Her thirst, polydipsia, pollakisuria, and polyuria resolved. The serum sodium and chloride levels, Posm, and Uosm also normalized. The patient was instructed to continue desmopressin therapy, was given information about an adequate daily water intake, and was discharged on day 28 of admission.
The patient’s subsequent clinical course has been uneventful for more than 3 years.
Discussion
The clinical features of diabetes mellitus in our patient, such as islet autoantibody negativity, relatively preserved endogenous insulin secretion capacity, comorbid hypertension, and dyslipidemia, and a family history of T2D supported the diagnosis of T2D [4,5]. Medical treatment with diet therapy and oral hypoglycemic agents controlled the hyperglycemia and its symptoms, and no chronic diabetic complications such as diabetic retinopathy occurred. However, 5 years after the T2D developed, the patient developed thirst, polydipsia, and polyuria, and was diagnosed with CDI. This case highlights the need for physicians to be aware of CDI as a rare but important comorbid disorder in patients with diabetes mellitus, including T2D, especially in those who present with thirst, polydipsia, and polyuria despite well-controlled glycemia.
Reported cases of co-existing CDI and T2D show different onset patterns at various ages. A 56-year-old man had simultaneous CDI and T2D with no abnormal brain MRI findings [12]. An obese 16-year-old boy developed T2D associated with nonketotic severe hyperglycemia 2 months after undergoing brain surgery for craniopharyngioma and developing hypopituitarism with CDI [13]. An obese 41-year-old man with a 5-year history of treated CDI developed T2D associated with hyperosmolar diabetic coma and was also diagnosed with Klinefelter syndrome [9]. There have also been a few reports of CDI that developed in patients being treated for T2D [10,11], as in our case.
Table 3 summarizes the characteristics of reported CDI cases that developed in patients with treated T2D. In a case of poorly controlled hyperglycemia, CDI was suspected due to worsening polyuria and a low urine specific gravity despite the presence of significant glucosuria, 10 years after the diagnosis of T2D [11]. In another case, in a patient with a 2-year duration of T2D, CDI was suspected because of recurring polyuria 6 weeks after correcting the hyperglycemia with insulin therapy [10]. Our patient initially developed T2D presenting with thirst, polydipsia, and polyuria. Medical treatment controlled the hyperglycemia and its symptoms. However, 5 years later, she re-exhibited thirst, polydipsia, and polyuria despite well-controlled glycemia and was diagnosed with CDI. Thus, regular good glycemic control not only relieved the hyperglycemic symptoms but also helped us to detect CDI early.
CDI, a deficiency of AVP, may be inherited or acquired [2,3,21]. Hereditary CDI is caused by mutations in the gene encoding AVP, and symptoms develop gradually at various ages during childhood [25]. Acquired CDI has many causes, such as traumatic brain injury, brain surgery, pituitary and hypothalamic tumors, and inflammatory, infiltrative, autoimmune, and vascular diseases [2,3,21,26]. However, many acquired cases are idiopathic with no identifiable cause [2]; in these idiopathic cases, autoimmunity may be an etiology [3], especially when other organ-specific autoimmune disorders are present [21,23]. In the present case, because the patient had a negative family history for CDI and developed CDI at an advanced age, her CDI was likely to have been acquired. No morphological abnormality in the intra- or suprasellar regions was detected on MRI, while she exhibited an autoimmune thyroid disease [24]. These findings suggested autoimmunity as a possible etiology for her idiopathic CDI.
The pathogenesis of T2D is also heterogeneous and involves many factors, including genetic and environmental ones, such as overeating, lack of physical activity, and resultant obesity [4,5]. Unlike type 1 diabetes mellitus, T2D is not typically associated with pancreatic autoimmunity. These findings suggest that there was no etiological link between our patient’s CDI and T2D.
Conclusions
We describe an older patient with a 5-year history of well-treated T2D who developed thirst, polydipsia, and polyuria and was diagnosed with CDI. Desmopressin therapy effectively relieved the symptoms. Physicians should be aware of CDI as a rare but important comorbid disorder in patients with diabetes mellitus, including T2D, especially in those who present with thirst, polydipsia, and polyuria despite well-controlled glycemia.
References:
1.. Baldeweg SE, Ball S, Brooke A, Society for Endocrinology Clinical Guidance: Inpatient management of cranial diabetes insipidus: Endocr Connect, 2018; 7; G8-G11
2.. Hui C, Khan M, Radbel JM, Diabetes insipidus: StatPearls [Internet] Aug 12, 2022, Treasure Island (FL), StatPearls Publishing 2022
3.. Tomkins M, Lawless S, Martin-Grace J, Diagnosis and management of central diabetes insipidus in adults: J Clin Endocrinol Metab, 2022; 107; 2701-15
4.. Seino Y, Nanjo K, Tajima N, Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus: J Diabetes Investig, 2010; 1; 212-28
5.. , 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020: Diabetes Care, 2020; 43(Suppl. 1); S14-31
6.. d’Annunzio G, Minuto N, D’Amato E, Wolfram syndrome (diabetes insipidus, diabetes, optic atrophy, and deafness): clinical and genetic study: Diabetes Care, 2008; 31; 1743-45
7.. Sakaguchi H, Sanke T, Ohagi S, A case of Albright’s hereditary osteodystrophy-like syndrome complicated by several endocrinopathies: Normal Gs alpha gene and chromosome 2q37: J Clin Endocrinol Metab, 1998; 83; 1563-65
8.. Bhan GL, O’Brien TD, Autoimmune endocrinopathy associated with diabetes insipidus: Postgrad Med J, 1982; 58; 165-66
9.. Isobe K, Niwa T, Ohkubo M, Klinefelter’s syndrome accompanied by diabetes mellitus and diabetes insipidus: Intern Med, 1992; 31; 917-21
10.. Paulose KP, Padmakumar N, Diabetes insipidus in a patient with diabetes mellitus: J Assoc Physicians India, 2002; 50; 1176-77
11.. Akarsu E, Buyukhatipoglu H, Aktaran S, Geyik R, The value of urine specific gravity in detecting diabetes insipidus in a patient with uncontrolled diabetes mellitus: Urine specific gravity in differential diagnosis: J Gen Intern Med, 2006; 21; C1-2
12.. Shin HJ, Kim JH, Yi JH, Polyuria with the concurrent manifestation of central diabetes insipidus (CDI) & type 2 diabetes mellitus (DM): Electrolyte Blood Press, 2012; 10; 26-30
13.. Soto AG, Cheruvu S, Bialo D, Quintos JB, Refractory diabetes insipidus leading to diagnosis of type 2 diabetes mellitus and non-ketotic hyperglycemia in an adolescent male: R I Med J, 2014; 97; 34-35
14.. Burmazovic S, Henzen C, Brander L, Cioccari L, One too many diabetes: The combination of hyperglycaemic hyperosmolar state and central diabetes insipidus: Endocrinol Diabetes Metab Case Rep, 2018; 2018; 18-0029
15.. Fraser CL, Arieff AI, Fatal central diabetes mellitus and insipidus resulting from untreated hyponatremia: A new syndrome: Ann Intern Med, 1990; 112; 113-19
16.. Masood M, Kumar S, Asghar A, Jabbar A, An unusual case of central diabetes insipidus & hyperglycemic hyperosmolar state following cardiorespiratory arrest: BMC Res Notes, 2013; 6; 325
17.. Inaba H, Funahashi T, Ariyasu H, Diabetic ketoacidosis in a patient with acromegaly and central diabetes insipidus treated with octreotide long-acting release: Diabetol Int, 2016; 8; 237-42
18.. Takano N, Yatabe MS, Yatabe J: BMC Infect Dis, 2018; 18; 363
19.. Zheng Y, Ley SH, Hu FB, Global aetiology and epidemiology of type 2 diabetes mellitus and its complications: Nat Rev Endocrinol, 2018; 14; 88-98
20.. Muhsin SA, Mount DB, Diagnosis and treatment of hypernatremia: Best Pract Res Clin Endocrinol Metab, 2016; 30; 189-203
21.. Christ-Crain M, Winzeler B, Refardt J, Diagnosis and management of diabetes insipidus for the internist: an update: J Intern Med, 2021; 290; 73-87
22.. Kurokawa H, Fujisawa I, Nakano Y, Posterior lobe of the pituitary gland: Correlation between signal intensity on T1-weighted MR images and vasopressin concentration: Radiology, 1998; 207; 79-83
23.. Hannon MJ, Orr C, Moran C, Anterior hypopituitarism is rare and autoimmune disease is common in adults with idiopathic central diabetes insipidus: Clin Endocrinol (Oxf), 2012; 76; 725-28
24.. Caturegli P, De Remigis A, Rose NR, Hashimoto thyroiditis: Clinical and diagnostic criteria: Autoimmun Rev, 2014; 13; 391-97
25.. Spiess M, Beuret N, Rutishauser J, Genetic forms of neurohypophyseal diabetes insipidus: Best Pract Res Clin Endocrinol Metab, 2020; 34; 101432
26.. Verbalis JG, Acquired forms of central diabetes insipidus: Mechanisms of disease: Best Pract Res Clin Endocrinol Metab, 2020; 34; 101449
Tables
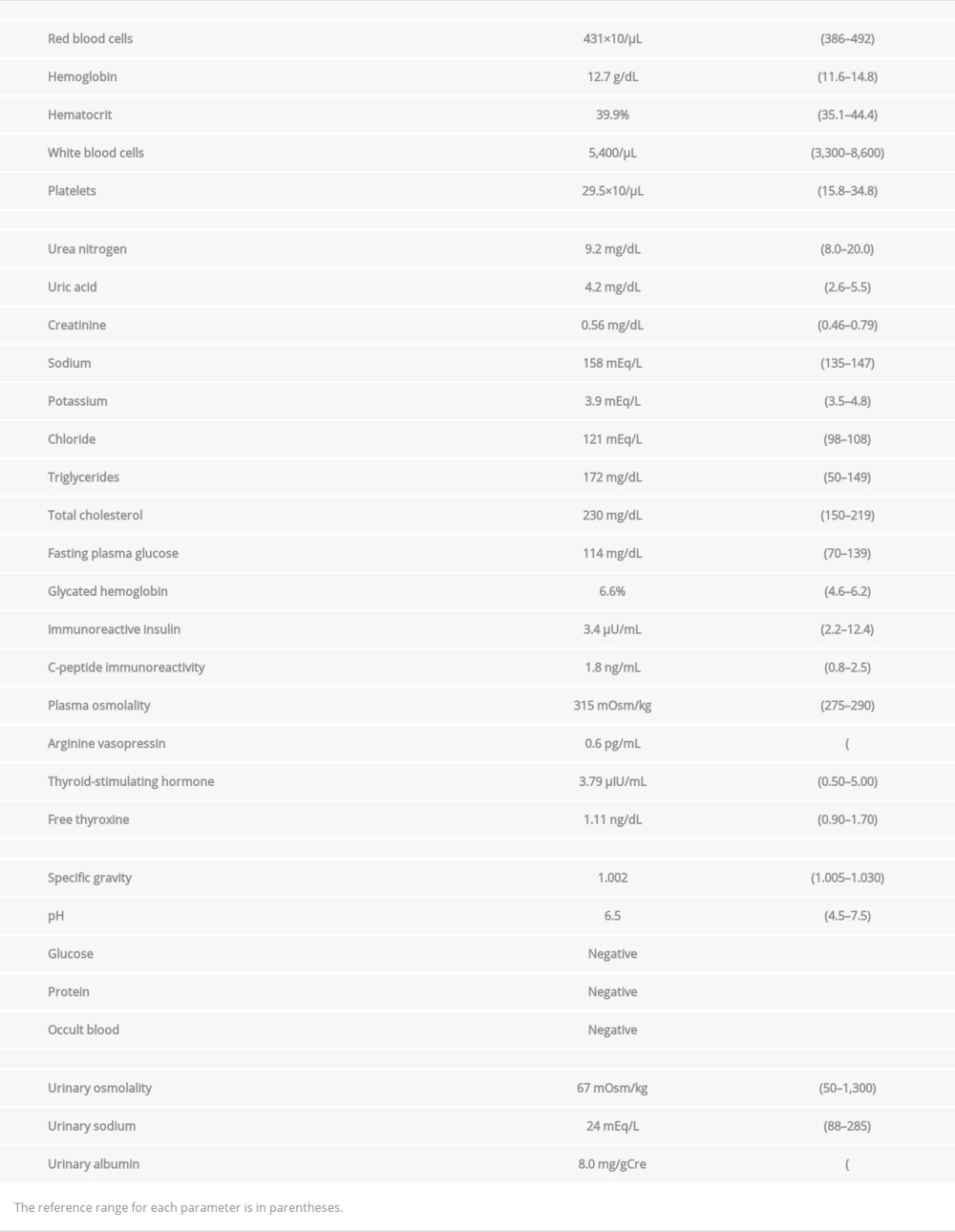 Table 1.. Laboratory test results.
Table 1.. Laboratory test results.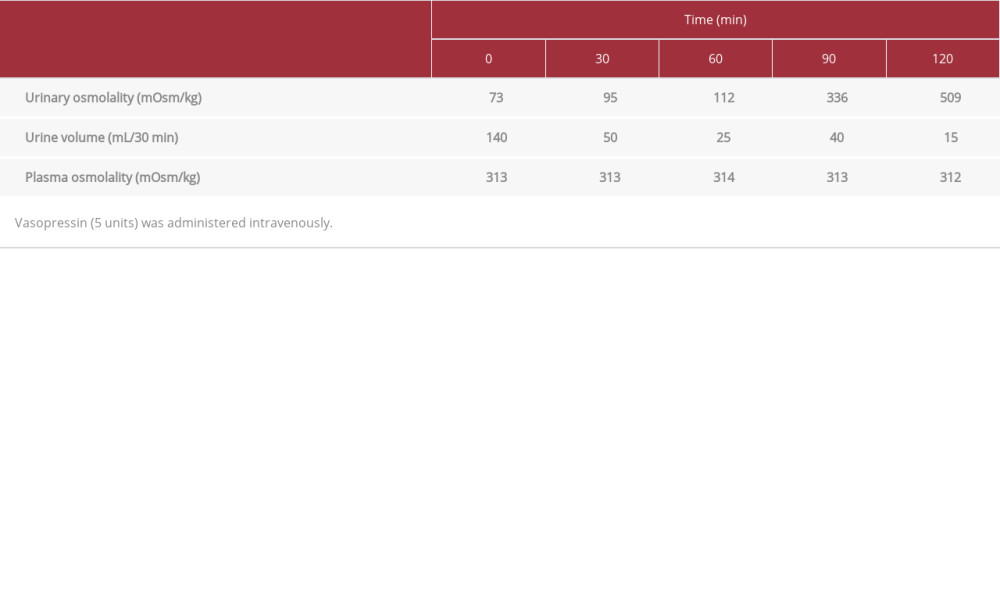 Table 2.. Vasopressin administration test results.
Table 2.. Vasopressin administration test results.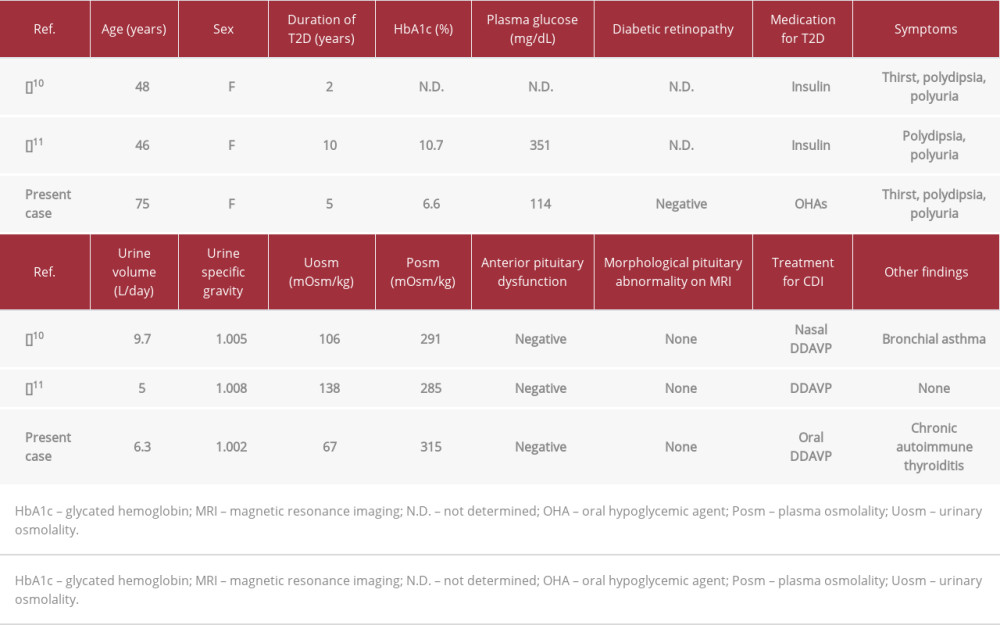 Table 3.. Summary of reported cases of central diabetes insipidus (CDI) that developed in patients with treated type 2 diabetes mellitus (T2D).
Table 3.. Summary of reported cases of central diabetes insipidus (CDI) that developed in patients with treated type 2 diabetes mellitus (T2D). Table 1.. Laboratory test results.
Table 1.. Laboratory test results. Table 2.. Vasopressin administration test results.
Table 2.. Vasopressin administration test results. Table 3.. Summary of reported cases of central diabetes insipidus (CDI) that developed in patients with treated type 2 diabetes mellitus (T2D).
Table 3.. Summary of reported cases of central diabetes insipidus (CDI) that developed in patients with treated type 2 diabetes mellitus (T2D). In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








