07 June 2023: Articles 
Rare Case of Refractory Hypokalemia in a Patient with Acute Monocytic Leukemia
Unknown etiology, Challenging differential diagnosis, Diagnostic / therapeutic accidents, Unusual setting of medical care
Minha Naseer1EF*, Brooke KaniaDOI: 10.12659/AJCR.938775
Am J Case Rep 2023; 24:e938775
Abstract
BACKGROUND: Refractory hypokalemia has been rarely demonstrated in patients with acute monocytic leukemia (AMoL). Hypokalemia develops in these patients owing to renal tubular dysfunction, secondary to lysozyme enzymes that are released by monocytes in AMoL. Additionally, renin-like substances are produced from monocytes and can lead to hypokalemia and metabolic alkalosis. There is also an entity called spurious hypokalemia, in which high numbers of metabolically active cells in blood samples increase sodium-potassium ATPase activity, resulting in influx of potassium. Additional research is warranted regarding this specific demographic to create standardized treatment approaches to electrolyte repletion.
CASE REPORT: In this case report, we demonstrate a rare case of an 82-year-old woman with AMoL, complicated by refractory hypokalemia, who presented with concerns of fatigue. The patient’s initial laboratory results were significant for leukocytosis with monocytosis and severe hypokalemia. Refractory hypokalemia was noted, despite administration of aggressive repletions. During her hospitalization, AMoL was diagnosed and an extensive workup was performed to evaluate the underlying cause of hypokalemia. Ultimately, the patient died on day 4 of hospitalization. We describe the correlation between severe refractory hypokalemia and leukocytosis and provide a literature review of multiple etiologies of refractory hypokalemia in patients with AMoL.
CONCLUSIONS: We evaluated the numerous pathophysiologic mechanisms responsible for refractory hypokalemia in patients with AMoL. Our therapeutic outcomes were limited owing to the patient’s early death. It is of high importance to evaluate the underlying cause of hypokalemia in these patients and to treat accordingly with caution.
Keywords: Hypokalemia, Leukemia, Aged, 80 and over, Female, Humans, Leukemia, Monocytic, Acute, Leukocytosis, Potassium
Background
Acute monoblastic/monocytic leukemia is considered a type of acute myeloid leukemia (AML). In order to fulfill the World Health Organization (WHO) criteria for this subtype of leukemia, a patient must have greater than 20% blasts in the bone marrow, and of these, greater than 80% must be of the monocytic lineage [1]. Refractory hypokalemia has been rarely demonstrated in patients with acute monocytic leukemia (AMoL). A diverse group of acid-base balance disorders and electrolyte abnormalities have been described in patients in which hypokalemia in AMoL may be multifactorial in origin. Hypokalemia is the most pronounced electrolyte abnormality in patients with AMoL. Its frequency ranges from 43% to 64% in this population [2]. This has been discovered in patients during disease relapse as well as during remission. This phenomenon has mainly been attributed to lysozymuria-induced renal tubular injury with kaliuresis and persists despite potassium supplementation [2]. Here, we present a case of an 82-year-old woman who presented with fatigue and subsequently received a diagnosis of AMoL and was found to have refractory hypokalemia as a consequence of the disease.
Case Report
An 82-year-old woman with no significant past medical history presented to the Emergency Department (ED) with a chief concern of fatigue for 2 weeks. An additional review of systems was positive for bilateral lower extremity edema and night sweats. The patient denied dyspnea, cough, chest pain, palpitations, paroxysmal nocturnal dyspnea, orthopnea, fever, chills, heat/cold intolerance, weight changes, appetite loss, hair loss, abdominal pain, vomiting, and diarrhea. She denied intake of any prescribed or herbal medications on a regular basis. Per the patient, she previously lived at home alone and endorsed the ability to complete her activities of daily living independently, which was difficult since the new onset of fatigue. In the ED, vital signs were within normal limits, including blood pressure of 136/88 mmHg and heart rate of 98 beats per min. Physical examination was significant for conjunctival pallor and splenomegaly. Electrocardiogram on admission showed normal sinus rhythm with some premature ventricular contractions, heart rate of 94, prolonged QTc of 596, T-wave flattening, and absence of ST-segment changes (Figure 1).
Laboratory studies were consistent with severe leukocytosis, with monocytosis, normocytic anemia, and thrombocytopenia (Table 1). Additional laboratory studies demonstrated hypokalemia, hypomagnesemia, hyperuricemia, and intrinsic acute kidney injury (blood urea nitrogen/creatinine ratio <20; Table 2). The patient’s baseline serum creatinine level was unknown.
The patient’s arterial blood sample was compared with the serum chemistry results to rule out pseudo-hypokalemia prior to initiating administration of potassium repletion. For repletion, the patient received a total of 130 mEq of intravenous (i.v.) potassium chloride, 80 mEq of oral potassium chloride, 30 mEq of i.v. potassium phosphate, 1000 mg of oral potassium phosphate, 4 tablets of oral Neutra-phos, 8 mEq of i.v. magnesium sulfate, and 400 mg of oral magnesium oxide over the course of her admission to treat her electrolyte disturbances.
The patient also received 1 dose of rasburicase, a uricolytic agent, and was started on allopurinol in anticipation of tumor lysis syndrome. In the meantime, investigations for the cause of hypokalemia revealed the results shown in Table 2. Urine electrolytes demonstrated a urine potassium to urine creatinine ratio of >1.5, with a urine potassium level of 44 mEq/L on day 3 and 56 mEq/L on day 4, indicating renal wasting while the patient was receiving potassium replenishment (Table 3). Repletions were not paused prior to the urine studies, which might have overestimated the renal potassium losses. The patient’s workup for hypokalemia, including lysozyme levels and renin-aldosterone studies, were negative (Table 4). Despite receiving aggressive replenishment of potassium and magnesium, the patient continued to experience persistent urinary losses of potassium.
The patient’s laboratory results raised suspicion of acute vs chronic leukemia, for which a bone marrow biopsy and further imaging was warranted. Ultrasound of the abdomen demonstrated splenomegaly. Computed tomography of the chest without contrast demonstrated small bilateral pleural effusions with adjacent opacities, most suggestive of atelectasis. Peripheral smear demonstrated abundant blast cells, with no auer rods seen. Flow cytometry study was suggestive of AMoL with 85% to 90%, CD14/CD64 blasts/immature monocytic elements, with downregulation of CD14, upregulation of CD2 and CD4, and aberrant expression of CD56 (Figure 2). The findings were compatible with AMoL. Immunostaining showed the leukemic cells were positive for muramidase and negative for myeloperoxidase, further supporting the diagnosis of AMoL.
Treatment options and diagnosis were discussed with the patient; however, the patient declined chemotherapy and decided to pursue palliative measures. The patient died on day 4 of hospitalization.
Discussion
Hypokalemia represents a common clinical problem. Potassium enters the body via oral intake, is stored within the cell, and is primarily excreted in the urine. Hypokalemia can be caused either by decreased intake or excessive losses of potassium through the urine, gastrointestinal tract, or sweat [3]. Potassium intake is normally 40 to 120 mEq per day, most of which is excreted via urine [3]. The kidney can reduce the excretion in case of hypokalemia to a minimum of 5 to 25 mEq per day [3]. Thus, low intake alone cannot represent the sole cause of hypokalemia but can only contribute to the severity of hypokalemia in the presence of other causes.
The most common causes of excessive excretion of potassium in the urine can result from the use of diuretic medications and excessive mineralocorticoid activity [4]. Less common etiologies of kaliuresis include genetic syndromes affecting the renal function, polyuria, and renal tubular acidosis. Gastrointestinal losses of potassium usually are due to long-standing diarrhea or vomiting, chronic laxative abuse, intestinal obstruction, or infections [4]. Concomitant magnesium deficiency has long been known to aggravate hypokalemia. More than 50% of patients with hypokalemia have concomitant hypomagnesemia, which is mostly seen in patients using diuretic therapy [4]. Our patient did not have any identifiable source of gastrointestinal loss of potassium.
Hypokalemia in AMoL is of a multifactorial origin. The exact pathogenic mechanism for this hyperkaluresis is unclear. One of the most common etiologies is lysozyme-induced renal tubular injury leading to persistent kaliuresis despite potassium supplementation [5]. Lysozyme (muramidase) is a small cationic enzyme, which is produced mainly by granulocytes and monocytes [5]. This enzyme commonly appears in normal tears, nasal secretions, sweat, serum, and urine [6]. High serum and urine lysozyme levels have been observed in patients with hyperproliferation of granulocytes, monocytes, and macrophages, including AML (primarily acute myelomonocytic leukemia and acute monoblastic/monocytic leukemia), multiple myeloma, sarcoidosis, and Hodgkin disease. [2–5]
Owing to its small size, lysozyme is filtered freely in the glomerulus and later reabsorbed in the proximal convoluted tubule. In cases of overproduction, this enzyme can accumulate in the tubular cells, allowing the kidney to act as a reservoir for circulating lysozyme [5]. When the load of lysozyme reaches above the threshold, this lytic enzyme becomes toxic to the tubular cells and leads to tubular cell damage, causing acute kidney injury and renal potassium wasting [5]. Patients with AMoL can have elevated serum and urine lysozyme levels, but a definitive diagnosis of lysosomal-induced nephropathy is made by kidney biopsy [6]. In our patient, serum lysozyme levels were within normal limits, thus excluding it as the cause of acute kidney injury and subsequent renal potassium wasting. Urinary lysozyme levels were not performed in our patient.
Renin is the initializing enzyme of the renin-angiotensin system, leading to the formation of angiotensin I and II. Another etiology of hypokalemia in patients with AMoL is paraneoplastic production of renin-like substances from monocytes [7]. It has been demonstrated that in some types of AML, especially acute myelomonocytic leukemia, blast cells, as compared with normal bone marrow cells, express significantly increased renin in the cytosol [7]. Activation of the renin-angiotensin-aldosterone system by paraneoplastic production of renin-like substances in AMoL blast cells can contribute to hypokalemia accompanied by metabolic alkalosis by affecting distal tubules of the kidney [7,8].
The cause of hypokalemia is usually apparent in a patient’s history, but if the cause is uncertain, further assessment of urine electrolyte studies and acid-base balance are performed to distinguish renal potassium loss from other causes [9]. A random urine potassium-creatinine ratio more than 1.5 mEq/mmol along with metabolic alkalosis suggests renal losses [9]. Our patient demonstrated a urine potassium-creatinine ratio of more than 1.5 mEq/mmol during aggressive repletion, indicating renal loss of potassium. However, potassium supplementation was not paused prior to these urinary studies, in anticipation of life-threatening complications of hypokalemia, including arrhythmias, which might have overestimated the renal loss of potassium.
Our patient presented with persistent hypokalemia and metabolic alkalosis. One of the possible etiologies for this metabolic derangement was thought to be damage to distal tubules, leading to increased reabsorption of sodium and excretion of potassium. Spot and 24-h urine studies showed increased excretion of potassium, despite persistent hypokalemia with normal spot urine sodium results, which can be misleading given our patient was being administered i.v. fluids. Metabolic alkalosis in our patient was likely secondary to an extracellular shift of K+ ions and intracellular shift of H+ to maintain electroneutrality. Similar derangements can be seen in patients with paraneoplastic production of aldosterone-like substances affecting distal tubules, leading to hypokalemia. This paraneoplastic production would lead to low aldosterone, which was seen in our patient as well.
There is also an entity called spurious hypokalemia, which can be seen in situations in which high numbers of metabolically active cells that are present in the blood sample absorb the extracellular fluid potassium. Pseudo-hypokalemia should always be considered in patients with leukocytosis secondary to hematological malignancies, especially when there are no clinical features to support these findings [10]. The inappropriate administration of potassium in such cases can cause serious cardiac arrhythmias [10]. A major mechanism is high activity of the Na+/K-ATPase pump, due to increased sodium permeability of leukemic cells, leading to increased shift of potassium into leukemic cells [10]. In our patient, severe hypokalemia was demonstrated in her serial arterial blood gas levels as well, which ruled out pseudo-hypokalemia as the blood samples of the arterial blood gases were placed on ice, and results were read before the blood was hemolyzed, elucidating the true potassium levels.
Treatment of potassium levels should be of importance in patients who are at risk of cardiac arrythmias and refractory hypokalemia [4]. Once the potassium level is below 3.5 mmol/L, treatment should be given regardless of the presence of symptoms [4,11]. Along with providing treatment, possible causes of potassium depletion should be addressed, including minimizing the dosage of potassium-wasting diuretics, restricting sodium and potassium intake [11]. Supplementations can be provided in i.v. and oral forms [4]. Oral forms of potassium include potassium chloride, potassium phosphate, and potassium bicarbonate [4]. Another factor that can affect potassium uptake and the maintenance of intracellular potassium levels is magnesium levels [11]. The role of magnesium in maintaining intracellular potassium is particularly important in cardiac myocytes, as this electrolyte desensitizes the myocytes to the calcium-induced arrhythmogenic actions of cardiac glycosides [11].
Oral potassium chloride raises potassium within 60 min and is cheaper and safer than giving i.v. potassium. Furthermore, larger quantities can be given with each dose. Thus, oral administration is usually preferred over the i.v. route. In patients who are unable to take orally administered potassium, and in the rare patient in whom more rapid correction of potassium is indicated, like our patient (serious arrhythmias, diaphragmatic paralysis resulting in respiratory failure, diabetic ketoacidosis, or resistant hypokalemia), potassium should be given intravenously [4].
Conclusions
Electrolyte disturbances in AMoL have multifactorial etiology and can present potential life-threatening arrhythmias and enhancement of the cardiotoxicity of specific chemotherapy agents received by patients with AMoL. Clinicians should be cautious and vigilant toward the early detection and appropriate treatment of concurrent hypokalemia in patients with AMoL prior to the initiation of and during treatment of chemotherapy. In this case of AMoL presenting with persistent hypokalemia, we evaluated the possible pathophysiologic mechanisms responsible for refractory hypokalemia in patients with AMoL. Our patient’s urine studies demonstrated excessive potassium loss; however, potassium repletion was not paused prior to these studies to avoid life-threatening complications of hypokalemia, including arrhythmias, which might have overestimated the renal loss of potassium. Paraneoplastic production of aldosterone would likely explain our patient’s metabolic derangement, although there are no reported cases with similar underlying mechanism of hypokalemia in patients with AMoL. This further emphasizes the importance of the additional research that is required on this topic to further explore other possible causes of hypokalemia in AMoL and appropriate treatment. Therapeutic outcomes are limited in our case, since our patient died shortly after presentation.
Figures
References:
1.. Hwang SM, Classification of acute myeloid leukemia: Blood Res, 2020; 55(S1); S1-S4
2.. Filippatos TD, Milionis HJ, Elisaf MS, Alterations in electrolyte equilibrium in patients with acute leukemia: Eur J Haematol, 2005; 75(6); 449-60
3.. Kardalas E, Paschou SA, Anagnostis P, Hypokalemia: A clinical update: Endocr Connect, 2018; 7(4); R135-46
4.. Tinawi M, Hypokalemia: A practical approach to diagnosis and treatment: Arch Clin Biomed Res, 2020; 4(2); 048-066
5.. Santoriello D, Andal LM, Cox R, Lysozyme-induced nephropathy: Kidney Int Rep, 2016; 2(1); 84-88
6.. Osserman EF, Lawlor DP, Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia: J Exp Med, 1966; 124(5); 921-52
7.. Wulf G, Jahns-Streubel G, Strutz F, Paraneoplastic hypokalemia in acute myeloid leukemia: A case of renin activity in AML blast cells: Ann Hematol, 1996; 73; 139-41
8.. Wulf G, Jahns-Streubel G, Nobiling R, Renin in acute myeloid leukemia blasts: Br J Hematol, 1998; 100; 335-37
9.. Assadi F, Diagnosis of hypokalemia: A problem-solving approach to clinical cases: Iran J Kidney Dis, 2008; 2(3); 115-22
10.. Chothia Y, Davids R, Pseudo-hypokalaemia and pseudo hypoxaemia in a patient with acute myeloid leukemia: Afr J Nephrol, 2020; 23; 15-18
11.. Cohn JN, Kowey PR, Whelton PK, Prisant LM, New guidelines for potassium replacement in clinical practice: A contemporary review by the National Council on Potassium in Clinical Practice: Arch Intern Med, 2000; 160(16); 2429-36
Figures
Tables
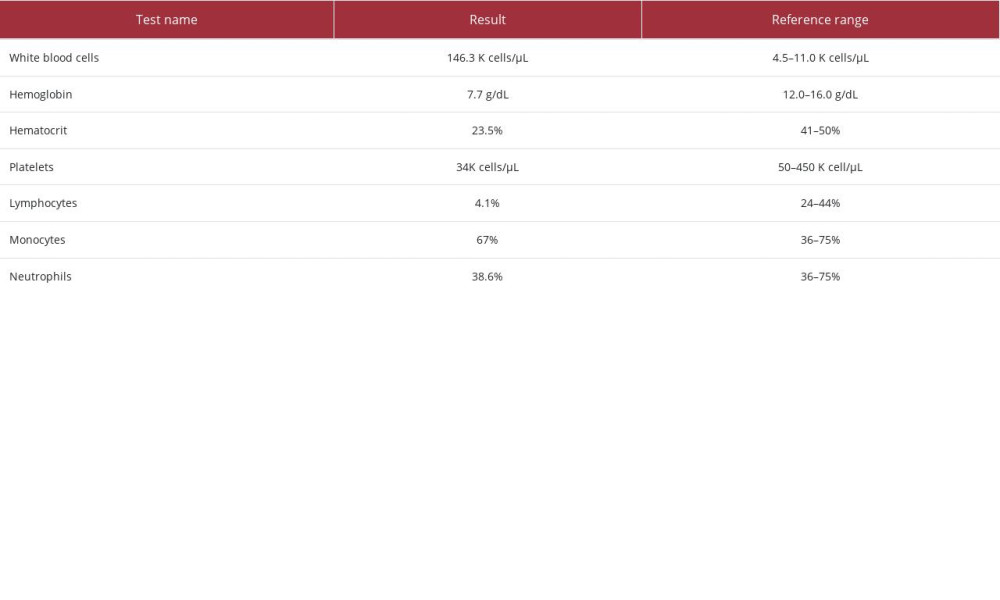 Table 1.. Complete blood count studies.
Table 1.. Complete blood count studies.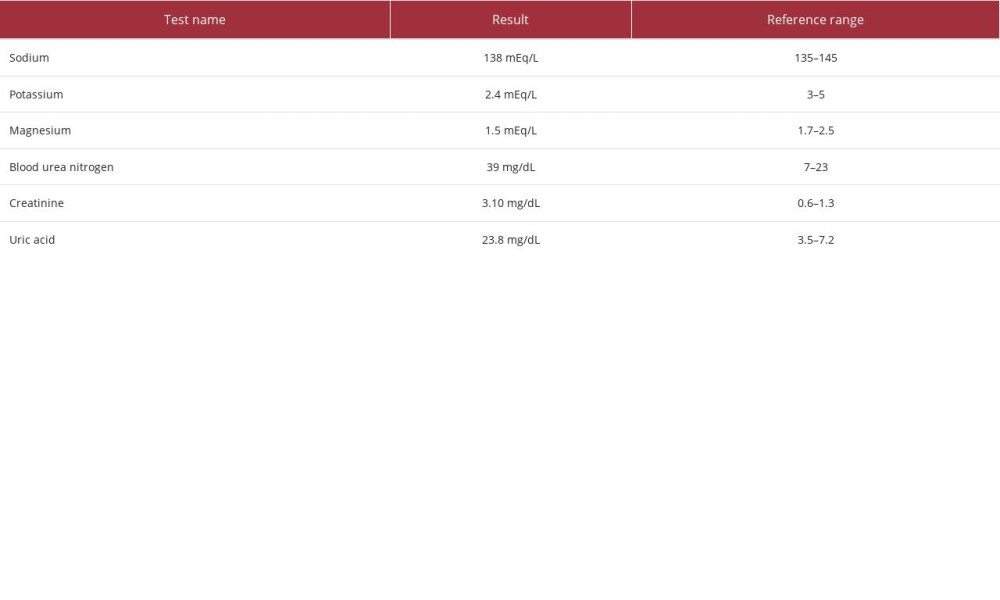 Table 2.. Serum chemistry studies.
Table 2.. Serum chemistry studies.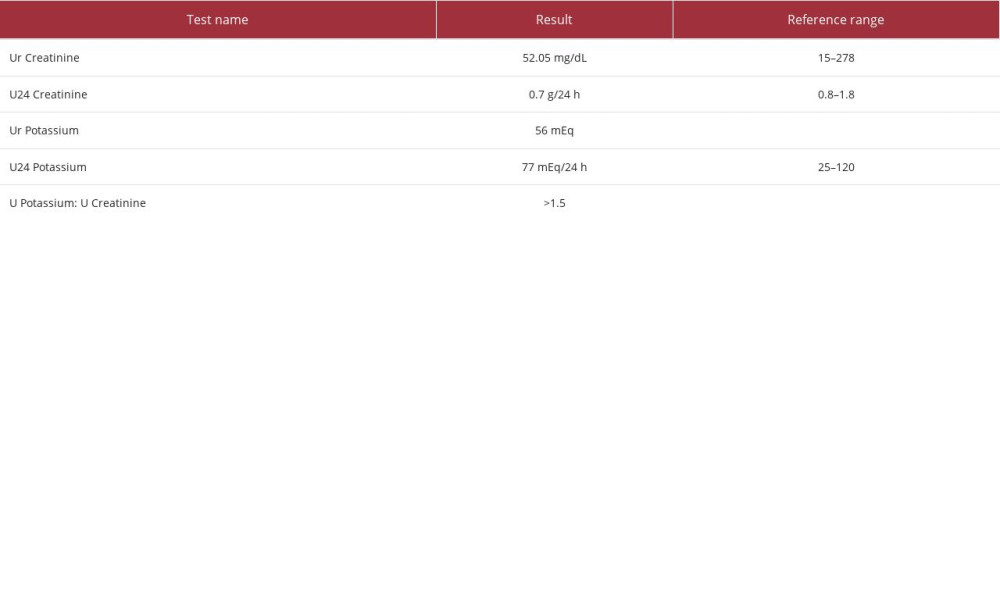 Table 3.. Urine studies.
Table 3.. Urine studies.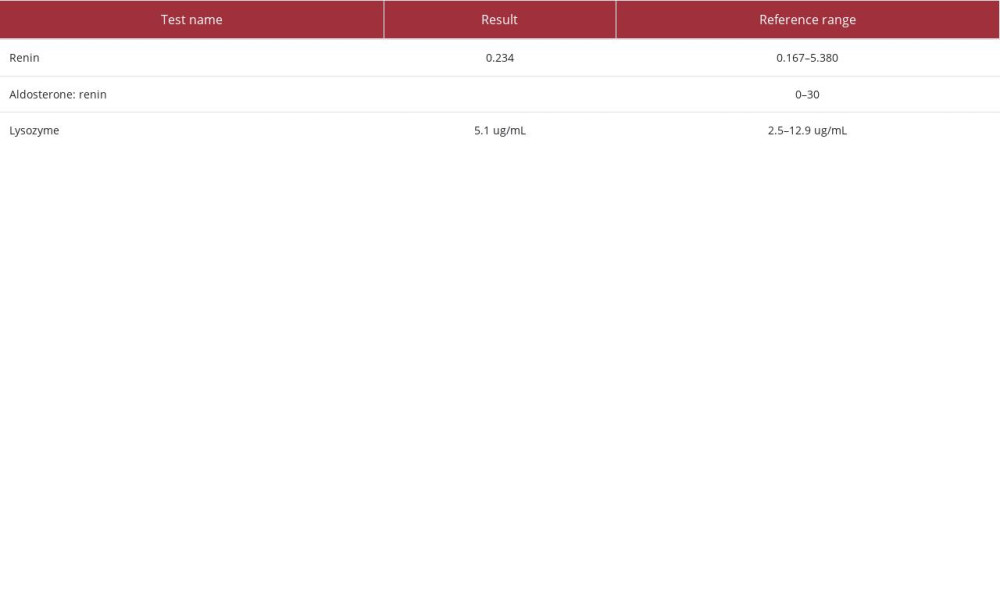 Table 4.. Additional laboratory studies contributing to workup for hypokalemia.
Table 4.. Additional laboratory studies contributing to workup for hypokalemia. Table 1.. Complete blood count studies.
Table 1.. Complete blood count studies. Table 2.. Serum chemistry studies.
Table 2.. Serum chemistry studies. Table 3.. Urine studies.
Table 3.. Urine studies. Table 4.. Additional laboratory studies contributing to workup for hypokalemia.
Table 4.. Additional laboratory studies contributing to workup for hypokalemia. In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








