12 April 2023: Articles 
Excision of a Benign Peripheral Giant Cell Granuloma in the Oral Mucosa of the Anterior Mandibular Teeth with a 975-nm Diode Laser: A Case Report of a 39-Year-Old Woman
Unknown etiology
Zana Sllamniku Dalipi1ABCDEFG, Mirlinda Sopi Krasniqi1AEF*, Labinota Kondirolli2CDOI: 10.12659/AJCR.938793
Am J Case Rep 2023; 24:e938793
Abstract
BACKGROUND: Peripheral giant cell granuloma, or epulis, is a common and benign oral lesion that can grow rapidly. Diode lasers are increasingly used to excise soft-tissue lesions because the technique preserves tissue for histopathology while controlling bleeding. Here, we report the excision of a 2-cm benign peripheral giant cell granuloma of the oral mucosa by 975-nm infrared diode laser, with rapid wound healing and good tissue preservation for histological analysis.
CASE REPORT: A 39-year-old woman presented with a large red-purple lesion in the oral mucosa of the lower jaw, near teeth 41 and 32. According to the patient, despite the absence of pain, the lesion caused difficulty while eating, speaking, and maintaining oral hygiene. The periodontal assessment included the following parameters: clinical attachment level, gingival recession, pocket probing depth, Loe-Silness gingival index, and tooth mobility index. The lesion was excised under local anesthesia using a 975-nm diode laser, and histopathology reports confirmed the diagnosis of peripheral giant cell granuloma. Six weeks after removal of the peripheral giant cell granuloma, all periodontal parameters were improved except for clinical attachment level and gingival recession.
CONCLUSIONS: Excision by 975-nm infrared diode laser can maintain tissue integrity for histopathology while allowing complete excision and control of bleeding. Soft lasers can provide advantages such as reduced bleeding, less operative and postoperative pain, decreased mechanical trauma, increased patient acceptability, and rapid wound healing without sutures, and they can be used to successfully remove peripheral giant cell granulomas.
Keywords: Low-Level Light Therapy, Granuloma, Giant Cell, Female, Humans, Adult, Mouth Mucosa, Lasers, Semiconductor, gingival recession, Pain, Postoperative
Background
Peripheral giant cell granuloma (PGCG) is an uncommon, benign, reactive, exophytic lesion of the gingiva in the oral cavity [1]. Clinically, the features of PGCG distinguish it from fibrous and vascular epulis. It presents as a soft, bright, pedunculated or sessile nodule with various sizes ranging from small papules to enlarged masses, sometimes exceeding 4 cm in diameter [2]; however, they are generally <20 mm in diameter, with a color that ranges from dark red to purple or blue [3]. Although PGCG occurs mostly in adults, some cases have been described in children, as in the case reported by Nekouei et al of a 4-year-old child with a lobular pedunculated soft-tissue mass in the left anterior maxilla [4].
PGCG has an unclear cause [5], but its formation is influenced by reactive lesions caused by local trauma or irritation [6], as well as other factors, including poor oral hygiene, chronic gingivitis, periodontal disease, and hormonal disorders [7]. PGCG is most frequently found in the interdental papillae region, more often on the vestibular surfaces of the intercanine region than on the oral surfaces; it is mainly located in the mandible [8,9].
There are no pathognomonic clinical features by which these lesions can be differentiated from other forms of gingival enlargement [10]. The differential diagnosis of PGCG includes pyogenic granuloma, peripheral ossifying fibroma, and peripheral cement-ossifying fibroma, all of which exhibit similar clinical and radiographic findings [10–12]. Very similar clinical and histological characteristics are present in central giant cell granuloma lesions [13]. Diagnoses of PGCG are based on histological analysis [14]. Radiographs are necessary to confirm the origin of giant cell lesions in the oral mucosa and to exclude central bony lesions with cortical perforation and soft-tissue extensions.
The standard course of treatment for PGCG involves surgical removal of the lesion and/or curettage of any bone defect [15]. Early detection of giant cell lesions permits more conservative surgery with lower risks of tooth and bone loss [16]. Surgical intervention should focus on eradicating the lesion from the base [17] because it is crucial to eliminate local factors that trigger PGCG; this approach reduces the risk of recurrence [18]. A recent meta-analysis revealed recurrence rates of ~9% over a mean follow-up period of around 2 years, suggesting that re-excision is necessary in a small percentage of patients [15]. Excision alone had a recurrence rate of 16%, while excision and curettage had a lower recurrence rate of 2.8%. Additionally, scaling and root planning may be required to eliminate periodontal pockets; in cases of severe bone resorption, tooth extraction may be necessary [19,20].
Recent research has shown that diode laser treatment can be used to eradicate oral soft-tissue hyperplasia; it may be preferred by patients, compared with conventional methods such as periodontal surgery and the use of caustic material [21]. Diode laser treatment has been successfully used to expose incisor and canine teeth; it has also been used to perform gingivectomy and frenectomy [22]. The use of a diode laser to treat oral soft-tissue enlargement has several advantages including less intraoperative bleeding, decreased postoperative pain, patient compliance, and more rapid wound healing [23,24]; however, it has been reported that diode lasers produce greater tissue damage compared with the conventional scalpel and the erbium, chromium-doped yttrium, scandium, gallium, and garnet lasers [25]. Here, we describe the short-term effects on periodontal parameters resulting from the use of a 975-nm diode laser to remove benign PGCG from the oral mucosa of the anterior mandibular teeth in a 39-year-old woman; we also describe the duration of wound healing and the quality of tissue preservation for histological analysis.
Case Report
A 39-year-old woman presented to the Periodontology and Oral Medicine Department at the University Clinical Dental Center in Pristina with a gingival growth in the region of the front teeth of the lower jaw. She denied having any allergies, systemic disease, or a history of tobacco or alcohol abuse. Laboratory investigation revealed a normal complete blood count. Orthopantomography showed a radiological lesion in the right mandibular body between both the lower first and second incisors, with d. 23 also impacted. However, intraoral clinical examination showed the absence of some teeth in the lower jaw, and the upper jaw contained a fixed prosthesis. The mucous membrane was healthy. The gingival tissue had begun to change 1 year previously in the form of bleeding during tooth brushing, as well as gingival hyperplasia. Because of gingival overgrowth and difficulty chewing, the patient requested functional improvement, along with esthetic improvement of the external gingiva. The gingival overgrowth was not associated with pain.
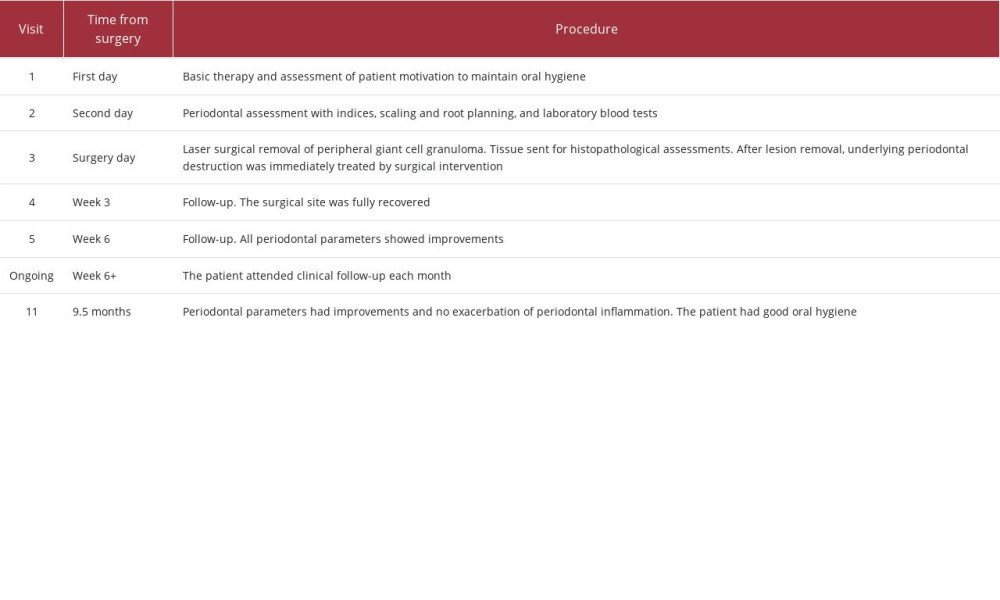
The lesion had a well-demarcated soft-tissue mass measuring 2 cm in diameter (as determined using a ruler), was red-purple in color, and localized in the region of teeth 41–32 in the vestibular site of the lower jaw (Figure 1; all images produced using a Galaxy Note20 Ultra 5G [Samsung, Suwon, South Korea]). It covered a substantial portion of the crown but did not cause any pain on palpation. Alveolar bone resorption was evident on oral panoramic X-rays between both the lower first and second incisors (Figure 2). Periodontal assessment included the following parameters: clinical attachment level (CAL) [26], gingival recession (GR) [26], pocket probing depth (PPD) [27], Loe-Silness gingival index (GI) [28], and tooth mobility index (TMI) [29]. These measurements were performed on 6 surfaces of the tooth using a Williams periodontal probe (CynaMed, Lorton, VA, USA) with divisions at 1, 2, 3, 5, 7, 8, and 9 mm. The PPD values were as follows: 3 mm for tooth 41, 3 mm for tooth 31, and 2 mm for tooth 32. The GR values for teeth 41, 31, and 32 were 4 mm, 3 mm, and 3 mm, respectively. For teeth 41, 31, and 32, the CAL values were 7 mm, 6 mm, and 5 mm, respectively. The TMI was 2 and GI was 2, indicating spontaneous gingival bleeding in the probed region. A complete list of the procedures used in this case is shown in Table 1.
We provided the patient with instructions for routine laboratory tests before treatment. Basic periodontal therapy and oral hygiene instruction were conducted, followed by root scaling and planning. Excision was performed using a diode laser (Hager & Werken, Duisburg, Germany) at 975 nm with a 400-nm optical fiber and local infiltrate anesthetic (Figure 3A, 3B). After lesion removal, the underlying periodontal destruction was immediately treated by surgical intervention.
After it had been fixed in 10% formalin [30], the excised lesion was sent for histopathological examination via hematoxylin and eosin staining. This examination revealed changes characterized by increased proliferation of fibroblasts, blood vessels, and lymphoplasmacytic infiltrates, along with numerous multinuclear giant cells beneath the acanthotic papillomatous squamous epithelium (Figure 4A, 4B). These findings supported a diagnosis of PGCG.
The patient was followed up at 1 and 3 weeks, then monthly thereafter. The surgical site fully recovered after 3 weeks. Six weeks after PGCG removal, all periodontal parameters showed improvements: for teeth 41, 31, and 32, the PPD, GI, and TMI values were 2 mm, 2 mm, and 2 mm, respectively. The CAL values were 6 mm, 6 mm, and 5 mm, whereas the GR values were 4 mm, 4 mm, and 3 mm for teeth 41, 31, and 32, respectively (Figure 5). At 9.5 months after PGCG removal, the periodontal parameters showed further improvements: for teeth 41, 31, and 32, the PPD, GI, and TMI values were [2] mm, [2] mm, and [2] mm, respectively. The CAL values were [6] mm, [6] mm, and [6] mm, whereas the GR values were [4] mm, [4] mm, and [4] mm for teeth 41, 31, and 32, respectively (Figure 6). Importantly, no recurrence of PGCG has been observed. The patient continues to undergo follow-up monitoring.
Discussion
The usual treatment for PGCG is excision down to the periosteum using a scalpel, followed by electrocautery, along with the confirmation that any local irritating factors are eliminated. Because of the high degree of vascularization of PGCG, surgical excision may require cauterization to avoid severe bleeding [4,8]. In the current case report, laser therapy was used for the surgical removal of PGCG localized in the region of the front teeth of the lower jaw to exploit the main advantages offered by this technique. These include the maintenance of sterility, reduction of bleeding, good estimation of cutting depth, precision of cutting, infrequent need for suturing or bandages, and pain reduction. This minimally invasive technique can reduce patient stress, promote wound healing, and result in less scarring than surgical excision [21]. Thus, patients can often resume routine activities more rapidly after laser surgery [31]. In this case, diode laser treatment was used to successfully remove PGCG in a patient, and wound healing was rapid (3 weeks) compared with the duration in cases treated via surgical excision (up to 10 months [15]). Additionally, the use of diode therapy did not interfere with histological analysis, in contrast to some previous reports [32].
Chen et al removed typical vascular epulis in the anterior teeth using a diode laser [33], with a favorable therapeutic outcome after a 5-year postoperative follow-up. Additionally, Ahn et al used diode laser treatment to expose incisor and canine teeth and to perform gingivectomy and frenectomy [22].
Furthermore, Cano-Durán et al showed that diode laser treatment of oral soft-tissue enlargement can facilitate improvements in intraoperative bleeding, postoperative pain, patient compliance, and wound healing [24].
Because of tissue thermal injury, diode laser surgery tends to result in more significant alterations than scalpel surgery [34]. Additionally, lasers are useful for soft-tissue surgery in modern dentistry, particularly in young patients, because they facilitate rapid wound healing without sutures [35]. Pregnancy epulis can be successfully treated with a diode laser, which preserves the affected teeth and causes less discomfort during treatment [36]. Rapid wound healing after laser surgery, which enables the patient to begin prosthetic rehabilitation sooner, is another advantage of laser treatment over open surgery [37]. Diode lasers can be used to treat oral soft-tissue lesions because they are simple to use, provide adequate coagulation, and cause minimal pain [38].
Multiple promising ancillary therapies (eg, probiotics, natural compounds, and ozonized water) have been shown to influence postoperative healing [39,40]. Future research could explore the benefits of diode laser surgery for PGCG in combination with these therapies.
There were some limitations in this case report. First, the follow-up duration was short. However, the focus of this preliminary report was not long-term recurrence; it was intended to describe short-term improvements in periodontal parameters after surgery, which may be practically useful for other clinicians. As noted above, we continue to monitor this patient. Second, these findings are based on the treatment of a single patient with a single treatment method; there was no comparison with other methods. Further studies in larger groups of patients, including randomized controlled trials, are needed to provide a more comprehensive assessment of this method and develop clinical recommendations with sufficient quality of evidence. Finally, these findings may not be generalizable to other patients with different clinical or demographic characteristics.
Conclusion
Excision by 975-nm infrared diode laser was sufficient to maintain tissue integrity for histopathology while allowing complete excision and control of bleeding. Considering the advantages of soft lasers (eg, reduced bleeding, operating time, and postoperative pain; decreased mechanical trauma; increased patient acceptability; and rapid wound healing without sutures), diode lasers may be used to successfully remove PGCGs.
Figures
References:
1.. Falaschini S, Ciavarella D, Mazzanti R, Peripheral giant cell granuloma: Immunohistochemical analysis of different markers. Study of three cases: Av Odontoestomatol, 2007; 23(4); 189-96
2.. Zeba J, Ahmad N, Shukla D, Diode laser for treatment of peripheral giant cell granuloma: J Dent Lasers, 2015; 9; 107-9
3.. Bodner L, Peist M, Gatot A, Fliss DM, Growth potential of peripheral giant cell granuloma: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1997; 83(5); 548-51
4.. Nekouei A, Eshghi A, Jafarnejadi P, Enshaei Z, A review and report of peripheral giant cell granuloma in a 4-year-old child: Case Rep Dent, 2016; 2016; 7536304
5.. Neville BW, Damm DD, Allen CM, Bouquot JE, Soft tissue tumors: Oral and Maxillofacial Pathology, 2009; 507-63, Louis, Saunders Publishers
6.. Tandon PN, Gupta SK, Gupta DS, Peripheral giant cell granuloma: Contemp Clin Dent, 2012; 3(Suppl. 1); S118-21
7.. Pacifici A, Carbone D, Marini R, Clinical management of a peri-implant giant cell granuloma: Case Rep Dent, 2015; 2015; 976756
8.. Shadman N, Ebrahimi SF, Jafari S, Eslami M, Peripheral giant cell granuloma: A review of 123 cases: Dent Res J (Isfahan), 2009; 6(1); 47-50
9.. Jané-Salas E, Albuquerque R, Font-Muñoz A, Pyogenic granuloma/ peripheral giant-cell granuloma associated with implants: Int J Dent, 2015; 2015; 839032
10.. Salum FG, Yurgel LS, Cherubini K, Pyogenic granuloma, peripheral giant cell granuloma and peripheral ossifying fibroma: Retrospective analysis of 138 cases: Minerva Stomatol, 2008; 57(5); 227-32
11.. Ogbureke EI, Vigneswaran N, Seals M, A peripheral giant cell granuloma with extensive osseous metaplasia or a hybrid peripheral giant cell granuloma-peripheral ossifying fibroma: A case report: J Med Case Rep, 2015; 9; 14
12.. Mishra AK, Maru R, Dhodapkar SV, Peripheral cemento-ossifying fibroma: A case report with review of literature: World J Clin Cases, 2013; 1(3); 128-33
13.. Rodrigues SV, Mitra DK, Pawar SD, Vijayakar HN, Peripheral giant cell granuloma: This enormity is a rarity: J Indian Soc Periodontol, 2015; 19(4); 466-69
14.. Costa P, Peditto M, Marcianò A, The “epulis” dilemma. Considerations from provisional to final diagnosis. A systematic review: Oral, 2021; 1; 224-35
15.. Chrcanovic BR, Gomes CC, Gomez RS, Peripheral giant cell granuloma: An updated analysis of 2824 cases reported in the literature: J Oral Pathol Med, 2018; 47(5); 454-59
16.. Flaitz CM, Peripheral giant cell granuloma: A potentially aggressive lesion in children: Pediatr Dent, 2000; 22(3); 232-33
17.. McDonald JS, Tumors of the oral soft tissues and cysts and tumors of bone: McDonald and Avery’s Dentistry for the Child and Adolescent, 2016; 606, St. Louis, Mo, Elsevier
18.. Ahlawat S, Gupta S, Rahman Siddiqui Z, Aziz Ikbal S, Peripheral giant cell granuloma: A case report: Asian J Oral Health and Allied Sci, 2022; 12; 6
19.. Derikvand N, Chinipardaz Z, Ghasemi S, Chiniforush N, The versatility of 980 nm diodelaser in dentistry: A case series: J Lasers Med Sci, 2016; 7(3); 205-8
20.. Pirnat S, Versatility of an 810 nm diode laser in dentistry: An overview: J Laser Health Acad, 2007; 4; 1-9
21.. Azma E, Safavi N, Diode laser application in soft tissue oral surgery: J Lasers Med Sci, 2013; 4(4); 206-11
22.. Ahn JH, Power S, Thickett E, Application of the diode laser for soft-tissue surgery in orthodontics: Case series: J Orthod, 2021; 48(1); 82-87
23.. Capodiferro S, Maiorano E, Loiudice AM, Oral laser surgical pathology: A preliminary study on the clinical advantages of diode laser and the histopathological features of specimens evaluated by conventional and confocal laser scanning microscopy: Minerva Stomatol, 2008; 57; 6-7
24.. Cano-Durán HA, Ortega-Concepción D, Peña-Cardelles JF, Employment of diode laser in the treatment of epulis fissuratum. case report: J Dent Med Sci, 2017; 16; 112-15
25.. Jin JY, Lee SH, Yoon HJ, A comparative study of wound healing following incision with a scalpel, diode laser or Er,Cr: YSGG laser in guinea pig oralmucosa: A histological and immunohistochemical analysis: Acta Odontol Scand, 2010; 68(4); 232-38
26.. Newman MG, Takei H, Klokkeveld PR, Carranza FA: Carranza’s Clinical Periodontology, 2014, St Louis, Mo, Elsevier
27.. Wilkins EM: Clinical practice of the dental hygienist; 1999, Philadelphia, Pa, Lippincott William and Wilkins
28.. Löe H, The gingival index, the plaque index and the retention index systems: J Periodontol, 1967; 38(6); 610-16
29.. Miller SC: Textbook of periodontia, 1950, Philadelphia, Pa, Blakiston
30.. Cloutier M, Charles M, Carmichael RP, Sándor GK, An analysis of peripheral giant cell granuloma associated with dental implant treatment: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2007; 103(5); 618-22
31.. Pandarathodiyil AK, Anil S, Lasers and their applications in the dental practice: Int J Dent Oral Sci, 2020; 7(11); 936-43
32.. Cercadillo-Ibarguren I, España Tost AJ, Arnabat Domínguez J, Histologic evaluation of thermal damage produced on soft tissues by CO2, Er,Cr: YSGG and diode lasers: Med Oral Patol Oral Cir Bucal, 2010; 15(6); 912-18
33.. Chen T-L, Wang X-M, Liu J, Therapeutic effects of diode laser on vascular epulis inesthetic area: J Indian Soc Periodontol, 2021; 25(1); 75-77
34.. D’Arcangelo C, Di Nardo Di Maio F, Prosperi GD, A preliminary study of healing of diode laser versus scalpel incisions in rat oral tissue: A comparison of clinical, histological, and immunohistochemical results: Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2007; 103(6); 764-73
35.. Ghadimi S, Chiniforush N, Najafi M, Amiri S, Excision of epulis granulomatosa with diode laser in 8 years old boy: A case report: J Lasers Med Sci, 2015; 6(2); 92-95
36.. Li L, Liu Y, 980-nm diode laser excision of a giant pregnancy epulis: Pak J Med Sci, 2022; 38(3 Part-I); 773-75
37.. Agarwal AA, Mahajan M, Mahajan A, Devhare S, Application of diode laser for excision of non-inflammatory vascular epulis fissuratum: Int J Case Rep Images, 2012; 3(9); 42-45
38.. Ortega-Concepción D, Cano-Durán JA, Peña-Cardelles JF, The application of diode laser in the treatment of oral soft tissues lesions. A literature review: J Clin Exp Dent, 2017; 9(7); e925-28
39.. Butera A, Gallo S, Maiorani C, Management of gingival bleeding in periodontal patients with domiciliary use of toothpastes containing hyaluronic acid, lactoferrin, or paraprobiotics: A randomized controlled clinical trial: Appl Sci, 2021; 11(18); 8586
40.. Butera A, Gallo S, Pascadopoli M, Ozonized water administration in peri-implant mucositis sites: A randomized clinical trial: Appl Sci, 2021; 11(17); 7812
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250