27 June 2023: Articles 
Inflammatory Arthritis After COVID-19: A Case Series
Challenging differential diagnosis, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Siddhant Yadav1ABCDEF*, Sara L. Bonnes1DEF, Elizabeth A. Gilman1DEF, Michael R. MuellerDOI: 10.12659/AJCR.939870
Am J Case Rep 2023; 24:e939870
Abstract
BACKGROUND: Some patients who have recovered from acute infection with SARS-CoV-2 develop persistent symptoms that have been termed post-COVID syndrome (PoCoS). PoCoS can affect the musculoskeletal system, with arthralgia and myalgia being common. Preliminary evidence suggests that PoCoS is an immune-mediated condition that not only predisposes but also precipitates pre-existing inflammatory joint diseases such as rheumatoid arthritis and reactive arthritis. Here, we describe a series of patients who presented to our Post-COVID Clinic and were found to have inflammatory arthritis (reactive and rheumatoid arthritis).
CASE REPORT: We present 5 patients who developed joint pain several weeks after recovery from acute SARS-CoV-2 infection. These patients were seen in our Post-COVID Clinic and came from locations across the United States. All 5 patients were women, with age of diagnosis of COVID-19 disease between 19 and 61 years (mean 37.8 years). All patients presented with joint pain as the primary concern to the Post-COVID Clinic. Abnormal joint imaging was present in all patients. Treatments varied and included non-steroidal anti-inflammatory drugs, acetaminophen, corticosteroids, immunomodulators (golimumab), methotrexate, leflunomide, and hydroxychloroquine.
CONCLUSIONS: COVID-19 disease is a potential cause of inflammatory arthritis, with both rheumatoid arthritis and reactive arthritis demonstrated in our PoCoS population. Care must be taken to identify these conditions, as there are treatment ramifications.
Keywords: COVID-19, Arthritis, Arthritis, Reactive, Arthritis, Rheumatoid, post-acute COVID-19 syndrome
Background
Like many other upper respiratory infections (URIs), infection with SARS-CoV-2, the virus that causes COVID-19 disease, can manifest with a variety of symptoms, including fevers, chills, arthralgia, myalgia, cough, nasal congestion, abdominal pain, nausea, and vomiting [1]. However, a significant number of patients have persistent symptoms after resolution of the acute phase of the infection. These persistent symptoms have been given a number of names, including post-acute sequelae of SARS-CoV-2 infection, long COVID-19, long haulers, and post-COVID syndrome (PoCoS) [2–4].
As with other viral URIs, musculoskeletal symptoms frequently occur during COVID-19 disease, with a spectrum of joint symptoms ranging from arthralgia to spurious and chronic arthritis [5,6]. The incidence of inflammatory arthritis in patients who have had COVID-19 is not known, but it has been established that viruses causing URIs (coronavirus, parainfluenza virus, influenza virus, metapneumovirus) coincide with an increased rate of development of rheumatoid arthritis (RA) [7]. Multiple studies have now reported autoantibodies in patients with COVID-19 disease (anti-cardiolipin, anti-beta 2 glycoprotein I, anti-citrullinated protein [CCP] antibody, rheumatoid factor) are associated with many autoimmune conditions, including Kawasaki-like disease, systemic lupus erythematosus, autoimmune hemolytic anemia, Guillain-Barre syndrome, and multiple sclerosis [8]. Although the mere presence of antibodies is not diagnostic of a disease state, the presence of these auto-antibodies does highlight the immune dysregulation caused by COVID-19 disease [9].
Reactive arthritis (ReA) is a disease process usually affecting patients under 50 years of age. The joint inflammation is usually preceded by a genitourinary infection (
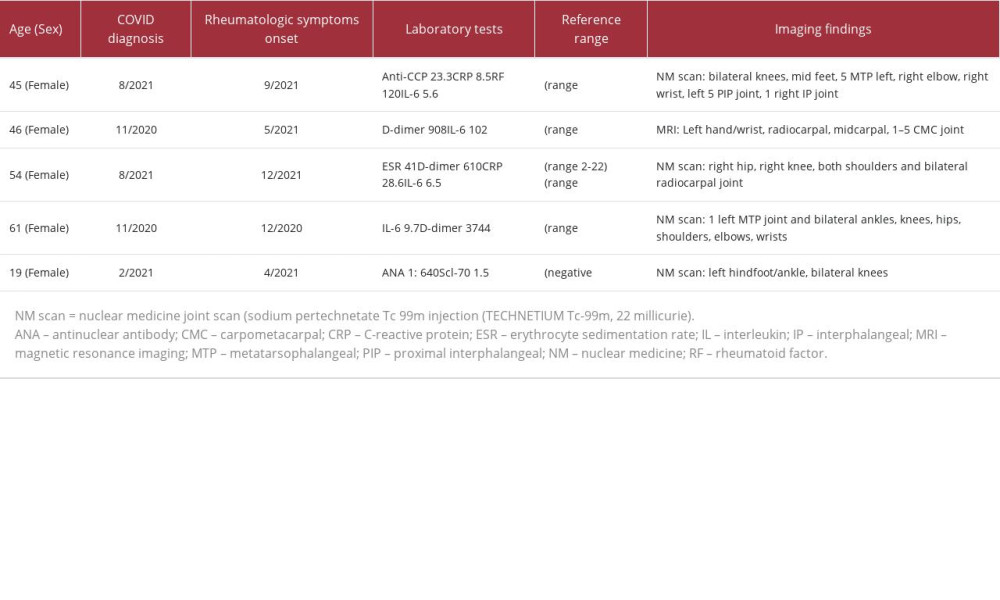
In this case series, we describe 5 patients (Table 1) who were evaluated in our Post-COVID-19 Care Clinic (PCOCC) and were found to have inflammatory arthritis, including ReA and RA.
Case Reports
CASE 1:
A 45-year-old highly functional woman with a significant past medical history of morbid obesity (body mass index 48 kg/m2), tobacco use, degenerative joint disease of the hip, and hip replacement developed anosmia and dysgeusia and tested positive for SARS-CoV-2 infection by polymerase chain reaction (PCR) in the fall of 2021. Her condition progressed and she developed fatigue, fever, and weakness, which eventually resolved after 3 weeks. She did not receive COVID-19-directed therapy. Rapid antigen testing 3 weeks later was negative.
Four weeks after her COVID-19 symptoms had abated, she developed sudden onset of severe right wrist pain and swelling. She went to the Emergency Department, where initially she was thought to have gout. She proceeded to develop joint pain in the knees, shoulders, and hands, with associated morning stiffness. Eventually, she was seen by a rheumatologist for her severe joint pain, which was causing sleep disturbance. On laboratory test results, a positive rheumatoid factor and elevated anti-CCP antibodies were noted. As a result, the patient was prescribed a course of steroids, which provided significant relief of her symptoms. She was trialed on methotrexate (unknown dosage); however, she had significant adverse effects, so she was transitioned to golimumab infusions (intravenously every 8 weeks) in February 2022. The golimumab infusions and the prednisone (10 mg daily) helped but did not resolve her symptoms completely. At the time of her visit to our PCOCC, 3 months after starting golimumab, she reported a 70% to 80% improvement in her symptoms.
On physical examination, she did not seem to be in any acute distress. The patient did endorse fatigue and a weight gain of more than 4.5 kg in the last few months, which was most likely related to the corticosteroid therapy. In her review of systems, she reported swelling in her legs and feet, palpitations, abdominal pain, constipation, arthralgia, back pain, pain and stiffness in the joints, joint swelling, and myalgia/muscle stiffness. The patient’s physical examination did not show her to have any clubbing, cyanosis, or edema in her extremities. There was no rash or suspicious lesion found on the skin examination. Although her joints did have genu valgus deformities, mild Heberden and Bouchard’s nodes were present. The patient did have tenderness of the right elbow, with resisted wrist flexion mainly along the lateral epicondyle. The bilateral knees had crepitus, with a decreased range of motion. There was no synovitis of the hands, wrists, elbows, shoulders, hips, knees, ankles, or toes. The patient’s vital signs were normal, with blood pressure of 125/79 mm Hg and pulse of 70 beats per min. She was afebrile. Her body mass index was 48.54 kg/m2.
A laboratory workup was significant, with a platelet count slightly elevated at 391/L (range 157–371), white blood cell count elevated at 10.0 (range 3.4–9.6), and lymphocytes elevated at 3.56/L (range 0.95–3.07). Her D-dimer was elevated at 1139 ng/mL (range <500), and her C-reactive protein (CRP) level was slightly elevated at 8.5 mg/L (range <8). Interestingly, her anti-CCP antibodies were slightly elevated at 23.3 units (range <20), her rheumatoid factor was substantially elevated at 120 international units (IU)/mL (range <15), and her interleukin (IL)-6 was elevated at 5.6 pg/mL (range <1.8). Her severe SARS-CoV-2 nucleocapsid antibody test was positive, consistent with prior SARS-CoV-2 infection.
Her nuclear medicine (NM) joint scan demonstrated increased periarticular radiotracer uptake involving the bilateral knees, bilateral mid feet, left fifth metatarsophalangeal joint, right elbow, left greater than right wrist, left fifth proximal interphalangeal joint, and the first right interphalangeal joint, consistent with synovitis (Figure 1). The patient was evaluated by our rheumatologist, who recommended the tapering of prednisone to better evaluate her symptoms of inflammatory arthritis. As per the last follow-up by our physician, the patient unfortunately continued to experience some of her long COVID symptoms but not specifically the arthritis.
CASE 2:
A 46-year-old previously healthy woman initially acquired COVID-19 disease in November 2020, which was characterized by an acute course of headaches, body aches, chills, and fatigue that lasted for 7 to 10 days and did not require any COVID-19-directed therapies or hospitalization. Approximately 6 to 8 weeks later, she began to experience upper thoracic back pain and chest pain. Computed tomography imaging of the chest demonstrated clear lungs and no concern for a pulmonary embolus. Of note, the patient was vaccinated with the Moderna vaccine, obtaining her 2 doses in February and April of 2021, along with a booster in November 2021. In the summer of 2021, 7 months after her first episode of COVID-19, she began to experience pain in her neck, jaw, left wrist, and legs. She subsequently developed bilateral Baker cysts, which ruptured in December 2021. In January 2022, she had her second bout of COVID-19, and the pain in her left wrist, both her knees, and legs became much more pronounced. She additionally reported a weight loss of 4.5 kg over the prior 6 months. Review of her outside medical records revealed a positive antinuclear antibody (ANA, 1: 320), with a negative extractable nuclear antibody panel, rheumatoid factor, and anti-CCP antibodies. The patient continued to have bilateral knee pain, with occasional swelling, right ankle pain, left wrist pain and swelling, upper thoracic/lower neck pain, and chest pain on inspiration. She denied skin rash, oral ulcers, alopecia, photosensitivity, malar erythema, dactylitis, red eyes, or proximal muscle weakness. Her symptoms were worse in the morning and improved with activities. Physical examination revealed left wrist swelling along with tenderness to palpation in the left wrist, as well as near the pes anserine bursa of both knees. She tried celecoxib (100 mg daily) and meloxicam (15 mg daily) without any benefit. She was also started on hydroxychloroquine 200 mg by a rheumatologist for the past 2 to 3 months for the possibility of seronegative RA, without significant benefit.
When she was seen at our institution in May 2022, she had an elevated D-dimer level of 908 ng/mL (range <500), elevated IL-6 level of 10.2 pg/mL (range <1.8), and negative anti-CCP antibodies, rheumatoid factor, and human leukocyte antigen (HLA) B27. An NM joint scan showed increased periarticular radiotracer uptake involving the bilateral knees, right ankle, and left wrist. Magnetic resonance imaging of her left hand and wrist showed marked enhancing synovitis with extensive effusions throughout the wrist, including the radiocarpal, midcarpal, and first to fifth carpometacarpal joints, associated with scattered joint erosions in the wrist and moderate enhancing tenosynovitis in the flexor compartment. Given her poor response to nonsteroidal anti-inflammatory drugs (NSAIDs) alone, these therapies were discontinued, and she was started on methotrexate 25 mg/mL injection weekly, along with folic acid 1 mg daily by our rheumatologists.
CASE 3:
A 54-year-old woman with no significant medical history developed flu-like symptoms in August 2021, associated with fatigue, body aches, loss of taste and smell, and a reduced appetite. She was never formally tested for COVID-19 but self-isolated at home for 10 days. In early September 2021, she developed abdominal pain, facial rash, and low back pain. Medical evaluation at that time was significant for elevated transaminase levels and steatosis on abdominal ultrasound. A liver biopsy in November 2021 showed “severe acute panacinar hepatitis with areas of parenchymal collapse with no definitive fibrosis identified”. Between November and December 2021, as her liver function levels were improving, she started developing significant joint pain and swelling. Her knees were most prominently affected, to the point where she needed to use crutches to walk. She was seen by her local rheumatologist, had a reportedly negative workup, and was started on prednisone 30 mg in January 2022. The follow-up erythrocyte sedimentation rate (ESR) and CRP level in March 2022 were in the normal range, along with a negative ANA result. Of note, the patient did receive COVID vaccination in April of 2022.
Her physical examination at our institution did not show any notable synovitis; however, she did have tenderness to palpation on both her hands, particularly on the right. Upon further evaluation at our institution in May 2022, she had an elevated ESR of 41 (range 2–22 mm/h), D-dimer level of 610 (range <500), CRP level of 28.6 (<8 mg/h), and IL-6 level of 6.5 (range <1.8). However, her ANA and anti-CCP antibody test results were both negative. Given the timing of her infection and clinical symptoms, her viral URI was thought to have been most likely COVID-19. An NM joint scan showed a mildly increased radiotracer uptake in the right hip, right knee, both shoulders, and bilateral radiocarpal joints, which was compatible with active inflammation. She was continued on her prednisone (40 mg daily), as it was providing some relief. The prednisone was tapered and eventually stopped later that month when her joint pain resolved. Two months later, she presented to the Rheumatology Clinic with concerns of right knee pain, which based on the evaluation, was likely mechanical in nature.
CASE 4:
A 61-year-old woman with type 1 diabetes mellitus and hypertension tested positive for COVID-19 in November 2020, with initial symptoms of fevers, chills, myalgia, anosmia, and dysgeusia. One month later, she developed post-exertional malaise and myalgia and arthralgia, predominantly in her hands, shoulders, and knees (more so on the right side). She additionally reported that the pain in her hands was associated with numbness. She trialed conservative therapy, including heat, ice, stretching, baths, ibuprofen, gabapentin, and opioids, but these therapies did not provide significant relief. She was evaluated by the Rheumatology Clinic, and her IL-6 level was elevated at 9.7 (range <1.8), and D-dimer level was elevated at 3744 (range <500), but the remainder of her inflammatory markers were normal. The NM joint scan showed increased radiotracer uptake in the first left metatarsophalangeal joint and bilateral ankles, knees, hips, shoulders, elbows, wrists, and hands, consistent with active inflammation. The patient was started on a trial of low-dose prednisone (15 mg/day), with significant improvement in pain in all her joints within a few days.
A trial of methotrexate and leflunomide as steroid-sparing agents was unsuccessful due to intolerable adverse effects. Hydroxychloroquine was tolerated, but prednisone was unable to be weaned below 10 mg/day due to increased pain and stiffness. At present, the patient continues on 10 mg of prednisone along with hydroxychloroquine, with good control of symptoms.
CASE 5:
A previously healthy 19-year-old woman was clinically diagnosed with COVID-19 in February 2021, with symptoms of dyspnea, lightheadedness, dizziness, chills, myalgia, congestion/rhinorrhea, nausea, vomiting, abdominal pain, and fatigue, which lasted for 3 weeks. Six to eight weeks later, she developed persistent joint pain in her bilateral knees, shoulders, ankles, hips, low back, neck, and wrists, which was in stark contrast to previous athletic training injuries that she rapidly recovered from.
Her physical examination in our clinic revealed her to have tenderness over her patellar tendon, but no synovitis was noted over her wrists or ankles. There was slight puffiness around her left ankle, with no notable tenderness. Her skin examination did not reveal any sclerodactyly or telangiectasia. When she was evaluated at our PCOCC, her ANA was elevated at 1: 640 (negative <1: 80) in a homogeneous pattern, and her Scl-70 level was slightly elevated at 1.5 (negative <1). The remainder of her laboratory test results were unremarkable, including antibodies to double-stranded DNA, complement, IL-6, HLA-B27, ESR (5 mm/h), CRP (<3 mg/L), and D-dimer. Her NM joint scan showed increased radiotracer uptake in her left hindfoot/ankle and bilateral knees, consistent with synovitis. The patient was seen in the Rheumatology Clinic, was started on corticosteroids, and had a rapid clinical response. She continues a low-dose tapering regimen of corticosteroid (methylprednisolone 4 mg daily) with her arthritic symptoms being kept at bay. Following this regimen, the patient states that this is the best that she has felt in years and now uses an NSAID (meloxicam 15 mg daily) as needed.
DIFFERENTIAL DIAGNOSIS:
The differential diagnosis for the patients in our case series with arthralgia and joint swelling after SARS-CoV-2 infection included osteoarthritis, viral polyarthritis, and autoimmune diseases, including ReA, RA, systemic lupus erythematosus, Sjogren syndrome, dermatomyositis, fibromyalgia, and mixed connective tissue disorder.
Even though all the symptoms for the patient in case 1 were not classical for RA, her inflammatory markers were elevated, and she had a positive rheumatoid factor and elevated anti-CCP antibodies, which were suggestive of RA [14]. Given her associated fatigue and poor sleep, the concern for chronic fatigue syndrome/fibromyalgia also arose, but she did not meet the diagnostic criteria for these syndromes. With the overlapping signs and symptoms of RA with various rheumatologic, dermatologic, endocrine pathologies, it is of the utmost importance to evaluate alternative diagnoses. Categorically ruling out alternative diagnoses with the help of serology tests and imaging is crucial.
To date, no validated diagnostic criteria or definitive laboratory test for ReA exists. The diagnosis of ReA is usually based on a clinical assessment following the exclusion of other differential diagnoses. In our cases 2, 3, 4, and 5, the diagnosis of ReA was made based on clinical findings and the exclusion of other inflammatory arthritis, based on blood workup and imaging (NM joint scan).
Discussion
Viral infections are a potential etiologic factor for the development of inflammatory arthritis and other forms of autoimmune arthritis, with multiple proposed pathophysiologic mechanisms [15]. These include (1) “molecular mimicry”, which occurs when a viral antigen mimics a host and activates cross reactive T cells; (2) “epitome spreading”, when tissue damage results from specific T-cell activation or direct virus-mediated host tissue destruction causing activation of autoreactive T cells and release of cell antigens into the inflammatory movement; (3) “super antigens” activating a wide range of nonspecific T cells; (4) “bystander activation” when autoreactive T cells are activated due to the release of cytokines when the virus targets the immune system; and (5) host antigens released from certain tissues during a virus-targeted immune response [7,8]. Additionally, local airway inflammation and neutrophil extracellular trap formation have been hypothesized as driving factors for anti-CCP antibody production in the lungs of first degree relatives with RA [16]. Neutrophil extracellular trap-derived proteases can cause the release of peptidylarginine deiminases, which could be pathogenic in RA. The presence of neutrophil extracellular traps has also been shown in the lung tissue and serum of patients with COVID-19 [17,18].
Genetic, environmental, hormonal, and immunologic factors have been implicated in the pathogenesis of RA and ReA. The strongest genetic risk factor for RA and ReA is the HLA class, accounting for 60% of genetic susceptibility for patients with RA and associated with a higher risk of chronicity [19,20]. Subsequently, the HLA-DRB 1 genotype is encountered in most cases of HLA-associated RA [21]. Analysis of HLA genotypes could prove useful in further work exploring the risk of inflammatory arthritis after COVID-19. Environmental factors, such as tobacco use, further increase the risk of immune reaction and production of anti-CCP antibodies [22]. Except for the patient in case 1, none of our other patients were current or previous users of tobacco in any form. The patient in case 1 used tobacco via traditional and electronic cigarettes, which both may have been predisposing to her inflammatory arthritis and contributing to its severity.
Our case series adds to a growing body of literature reporting the development of RA or ReA following COVID-19 [1,10,11,13,19,23–25]. In these cases, the severity of COVID-19 disease ranged from asymptomatic to critical, suggesting that there is no relationship between COVID-19 severity and arthritis risk. This relationship is similar to that seen in PoCoS, in which, while there is a higher incidence of PoCoS in patients with severe COVID-19, most cases seen had mild or moderate COVID-19, owing to the sheer number of patients in this category [26]. In our case series, our patients would be classified as having had mild or moderate COVID-19, as they did not require admission or supplemental oxygen.
ReA has been associated with gastrointestinal and genitourinary infections. Notably, gastrointestinal manifestations have been reported in upwards of 12% of COVID-19 patients [27,28]. It has been surmised that the gastrointestinal system may serve as a secondary site for coronavirus due to the expression of angiotensin converting enzyme-2 receptors in the gastrointestinal tract, which would support its role in the development of ReA [29]. Thus far, no genitourinary manifestations have been clearly associated with SARS-CoV-2 infection; thus, it is difficult to establish a plausible molecular mechanism for ReA by this pathway.
The rheumatoid factor level in the patient in case 1, checked 8 months after her SARS-CoV-2 infection, was markedly elevated at 120 IU, and her anti CCP levels were mildly elevated at 23.3 U. While her rheumatoid factor and anti-CCP antibody levels prior to the SARS-CoV-2 infection had not been tested, they continued to increase over 2 time points after SARS-CoV-2 infection. In the case report by Perrot et al, their patient’s anti-CCP antibody levels were already detectable when the patient had tested positive for SARS-CoV-2; however, her anti-CCP titers had increased substantially since the time she developed arthritis. It was postulated that this increasing anti-CCP titer trend was indicative of the spread of the epitope prior to the onset of clinical joint disease, suggesting that COVID-19 might have precipitated an initial flare of RA in their patient [25]. Unfortunately, in the case of ReA, no existing diagnostic test can be performed to follow disease activity, apart from following clinical symptoms over time. There is a possibility that inflammatory arthritis in our case series could have been coincidentally related to the SARS-CoV-2 infection; however, the timeline, whereby one of our patients continued to have elevated rheumatoid factor and anti-CCP levels even 8 months after the infection had subsided, might speak to the fact that the arthritic process could have been precipitated by the SARSCoV-2 infection. We do acknowledge that whereas not every case of inflammatory arthritis (ReA or RA) that is diagnosed after SARS-CoV-2 infection is necessarily related to the SARSCoV-2 infection, there is a theory that SARS-CoV-2 infection could be resulting in an inflammatory response, thereby resulting in a flare of arthritis in these patients [25].
Synovial fluid examination was not performed in any of our patients. In the case report by Gasparotto et al, synovial fluid analysis revealed inflammatory cells with polymorphonuclear predominance, with a negative RT-PCR for COVID-19, further supporting the hypothesis that this is an immune-mediated process [30]. Similarly, in the case reported by Lim et al, in their patient with ReA, synovial fluid analysis was negative for crystals, Gram stain,
Terracina et al report on a 55-year-old man with well-controlled non-erosive, seropositive RA at 2 years of clinical remission who developed an acute flare of his RA subsequent to the second dose of his mRNA COVID-19 vaccine. The authors postulated that this flare was secondary to an immune response to a component of the vaccine. The vaccine contained mRNA encoding for the SARS-CoV-2 spike protein plus other components that stabilize the vaccine in the circulation and promote its uptake into the cell by endocytosis. It was speculated that one of these components might have had a nonspecific adjuvant effect, or there could have been molecular mimicry between the viral spike protein and the patient’s immunoglobulins, resulting in the symptom flare [32–34]. Given the immune pathophysiology of ReA, it is likely that susceptible individuals could flare similarly after vaccination.
Inflammatory arthritis was identified in our patients via an increased uptake of the radioactive isotope on NM joint scans. In the existing literature, imaging studies in patients with inflammatory arthritis demonstrate findings suggestive of non-specific inflammation in the affected joints, including synovial tissue thickening, increased vascularity, and joint effusion [35].
Inflammatory changes have also been demonstrated on 14-fluorodeoxyglucose positron emission tomography/computed tomography, both in the joints [19] and the brain [3] in patients with PoCoS.
Conclusions
Persistent arthritis after COVID-19 has been referred to as post-COVID arthritis, post-viral arthritis, and post-COVID hyperinflammatory syndrome [35]. Irrespective of the nomenclature, the impact of COVID-19 as a potential cause of inflammatory arthritis must be recognized by clinicians. A detailed analysis of epidemiological, clinical, and serologic characteristics is needed for physicians to diagnose inflammatory arthritis, especially ReA and RA. The use of NM scans can provide useful information not only to diagnose but also to follow up inflammation in various joints in such patients, particularly in ReA, in which serum biomarkers are not currently available. Further studies are necessary to understand the pathogenesis of COVID-19 and its relation to arthritis by analyzing the presence of both the virus and the antibodies in the affected patient’s serum and synovial fluid, along with the incidence and evolution of the inflammatory manifestation.
References:
1.. Logue JK, Franko NM, McCulloch DJ, Sequelae in adults at 6 months after COVID-19 infection: JAMA Netw Open, 2021; 4(2); e210830
2.. Bierle DM, Aakre CA, Grach SL, Central sensitization phenotypes in post acute sequelae of SARS-CoV-2 infection (PASC): Defining the post COVID syndrome: J Prim Care Community Health, 2021; 12 21501327211030826
3.. Grach SL, Ganesh R, Messina SA, Hurt RT, Post-COVID-19 syndrome: Persistent neuroimaging changes and symptoms 9 months after initial infection: BMJ Case Rep, 2022; 15(4); e248448
4.. Soriano JB, Murthy S, Marshall JC, A clinical case definition of post-COVID-19 condition by a Delphi consensus.: Lancet Infect Dis, 2022; 22(4); e102-e07
5.. Slouma M, Mhemli T, Abbes M, Rheumatoid arthritis occurring after coronavirus disease 2019 (COVID-19) infection: Case based review: The Egyptian Rheumatologist, 2022; 44(3); 275-78
6.. Schett G, Manger B, Simon D, Caporali R, COVID-19 revisiting inflammatory pathways of arthritis: Nat Rev Rheumatol, 2020; 16(8); 465-70
7.. Joo YB, Lim YH, Kim KJ, Respiratory viral infections and the risk of rheumatoid arthritis: Arthritis Res Ther, 2019; 21(1); 199
8.. Shah S, Danda D, Kavadichanda C, Das S, Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment: Rheumatol Int, 2020; 40(10); 1539-54
9.. Ghozzi M, Melayah S, Adaily N, Ghedira I, Frequency of serological markers of rheumatoid arthritis in adult patients with active celiac disease: J Clin Lab Anal, 2022; 36(3); e24249
10.. Hannu T, Reactive arthritis: Best Pract Res Clin Rheumatol, 2011; 25(3); 347-57
11.. Honge BL, Hermansen MF, Storgaard M, Reactive arthritis after COVID-19: BMJ Case Rep, 2021; 14(3); e241375
12.. Parisi S, Borrelli R, Bianchi S, Fusaro E, Viral arthritis and COVID-19: Lancet Rheumatol, 2020; 2(11); e655-e57
13.. Baimukhamedov C, Barskova T, Matucci-Cerinic M, Arthritis after SARSCoV-2 infection.: Lancet Rheumatol., 2021; 3(5); e324-e25
14.. Fraenkel L, Bathon JM, England BR, 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis.: Arthritis Care Res (Hoboken), 2021; 73(7); 924-39
15.. Trovato CM, Montuori M, Pietropaoli N, Oliva S, COVID-19 and celiac disease: A pathogenetic hypothesis for a celiac outbreak: Int J Clin Pract, 2021; 75(9); e14452
16.. Demoruelle MK, Wang H, Davis RL, Anti-peptidylarginine deiminase-4 antibodies at mucosal sites can activate peptidylarginine deiminase-4 enzyme activity in rheumatoid arthritis: Arthritis Res Ther, 2021; 23(1); 163
17.. Arisan ED, Uysal-Onganer P, Lange S, Putative roles for peptidylarginine deiminases in COVID-19: Int J Mol Sci, 2020; 21(13); 4662
18.. Radermecker C, Detrembleur N, Guiot J, Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19: J Exp Med, 2020; 217(12); e20201012
19.. Coath FL, Mackay J, Gaffney JK, Axial presentation of reactive arthritis secondary to COVID-19 infection: Rheumatology (Oxford), 2021; 60(7); e232-e33
20.. Rai A, Aashish , Priya , Prevalence of rheumatoid arthritis following COVID-19 vaccine: An autoimmune disorder: Ann Med Surg (Lond), 2022; 82; 104628
21.. Sandoughi M, Fazaeli A, Bardestani G, Hashemi M, Frequency of HLA-DRB1 alleles in rheumatoid arthritis patients in Zahedan, southeast Iran: Ann Saudi Med, 2011; 31(2); 171-73
22.. Yap HY, Tee SZ, Wong MM, Pathogenic role of immune cells in rheumatoid arthritis: Implications in clinical treatment and biomarker development: Cells, 2018; 7(10); 161
23.. Derksen V, Kissel T, Lamers-Karnebeek FBG, Onset of rheumatoid arthritis after COVID-19: Coincidence or connected?: Ann Rheum Dis, 2021; 80(8); 1096-98
24.. Derksen VFAM, Kissel T, Lamers-Karnebeek FBG, Onset of rheumatoid arthritis after COVID-19: Coincidence or connected?: Ann Rheum Dis, 2021; 80(8); 1096-98
25.. Perrot L, Hemon M, Busnel JM, First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection: Lancet Rheumatol, 2021; 3(1); e6-e8
26.. Subramanian A, Nirantharakumar K, Hughes S, Symptoms and risk factors for long COVID in non-hospitalized adults: Nat Med, 2022; 28(8); 1706-14
27.. Hallak J, Teixeira TA, Bernardes FS, SARS-CoV-2 and its relationship with the genitourinary tract: Implications for male reproductive health in the context of COVID-19 pandemic: Andrology, 2021; 9(1); 73-79
28.. Parasa S, Desai M, Thoguluva Chandrasekar V, Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis: JAMA Netw Open, 2020; 3(6); e2011335
29.. Gheblawi M, Wang K, Viveiros A, Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2: Circ Res, 2020; 126(10); 1456-74
30.. Gasparotto M, Framba V, Piovella C, Post-COVID-19 arthritis: A case report and literature review: Clin Rheumatol, 2021; 40(8); 3357-62
31.. Liew IY, Mak TM, Cui L, A case of reactive arthritis secondary to coronavirus disease 2019 infection: J Clin Rheumatol, 2020; 26(6); 233
32.. Al-Allaf AW, Neethu M, Al-Allaf Y, A Case series and literature review of the association of COVID-19 vaccination with autoimmune diseases: Causality or chance?: Cureus, 2022; 14(9); e28677
33.. Dawoud R, Haddad D, Shah V, COVID-19 vaccine-related arthritis: A descriptive study of case reports on a rare complication: Cureus, 2022; 14(7); e26702
34.. Terracina KA, Tan FK, Flare of rheumatoid arthritis after COVID-19 vaccination: Lancet Rheumatol, 2021; 3(7); e469-e70
35.. Chaudhry ZS, Nellessen N, Reis C, Sharip A, The development of inflammatory arthritis following SARS-CoV-2 infection: A systematic review of the literature: Fam Pract, 2022; 39(6); 1116-34
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250