19 April 2024: Articles 
Simultaneous Transcatheter Closure of a Ventricular Septal Defect and Pulmonary Valvuloplasty: A Case Report
Unusual or unexpected effect of treatment, Congenital defects / diseases
Baraa AlghalyiniDOI: 10.12659/AJCR.942032
Am J Case Rep 2024; 25:e942032
Abstract
BACKGROUND: Congenital heart diseases (CHDs) are the most common form of birth defects, affecting the structure and function of neonatal hearts. Pulmonary valve stenosis (PVS) and ventricular septal defects (VSD) are 2 of the more prevalent forms, both of which can lead to significant morbidity if left untreated. The emergence of transcatheter techniques has revolutionized the therapeutic landscape, presenting minimally invasive yet effective alternatives to open-heart surgery and significantly reducing associated patient morbidity and recovery time.
CASE REPORT: The presented case details the management of a 19-year-old man with complex CHDs, highlighting the nuanced decision-making process that led to a transcatheter approach. The patient’s clinical presentation, marked by symptoms reflective of significant cardiac compromise, demanded a tailored approach that utilized the latest advancements in non-surgical intervention. The successful closure of the VSD with an Amplatzer device and the resolution of PVS via balloon valvuloplasty were achieved without complications, showcasing the potential of these techniques in managing similar cases. The post-intervention period was marked by a noteworthy recovery, confirming the procedural efficacy and enhancing the patient’s quality of life.
CONCLUSIONS: The favorable outcome of this case highlights the pivotal role of transcatheter interventions in treating complex CHDs and suggests a shift towards less invasive approaches in cardiac care. This case contributes valuable insights to the existing body of evidence, reinforcing the potential of transcatheter techniques to become the preferred treatment modality. With promising immediate and short-term results, these techniques highlight the need for continued research into their long-term efficacy and application across diverse patient demographics.
Keywords: atrioventricular septal defect, Balloon Valvuloplasty, Pulmonary Valve Stenosis, Septal Occluder Device
Introduction
Congenital heart diseases (CHDs) encompass a spectrum of functional and structural heart abnormalities present at birth, resulting from disruptions in embryonic organogenesis [1]. These conditions manifest in various forms, including alterations in the cardiac chambers, valves, or even the major blood vessels, which could potentially disrupt a patient’s normal blood flow [2]. Furthermore, individuals with CHDs can display a diversity of signs and symptoms, although these are frequently non-specific. If these conditions are left untreated, patients can develop severe complications such as heart failure, arrhythmia, or valvular insufficiency, leading to a significant increase in their mortality risk [3].
Pulmonary valve stenosis (PVS) and ventricular septal defect (VSD) are common types of CHDs. Congenital PVS is characterized by thickening or fusion of the pulmonary valve. Transcatheter pulmonary balloon valvuloplasty is a less invasive procedure than surgical valvuloplasty [4–6]. Also, trans-catheter closure of some types of VSDs is an established treatment modality [7].
Recent advances in medical technology have popularized the use of transcatheter interventions for managing congenital heart diseases. Such interventions are less invasive and are associated with lower morbidity and mortality rates compared to traditional surgical options [8–10]. This case report is a testament to the effectiveness of these interventions in managing such complex cases, further enriching the existing literature.
Case Report
PRESENTATION AND INITIAL ASSESSMENT:
A 19-year-old previously healthy Saudi man presented to our institution with atypical chest pain, shortness of breath, exercise intolerance, and palpitations, which were progressively limiting his physical activity. He denied any history of tobacco smoking, alcohol consumption, or illicit drug use. Physical examination revealed tachypnea with a respiratory rate of 20 breaths per minute and normal oxygen saturation of 95% on room air. His blood pressure was 124/80 mmHg with a regular heart rate of 90 beats per minute. Cardiovascular examination was significant for auscultatory findings suggestive of a ventricular septal defect and pulmonary stenosis, characterized by specific murmurs, without any evidence of peripheral edema.
DIAGNOSTIC INVESTIGATIONS:
Electrocardiography depicted right superior axis deviation, pulmonary disease pattern, incomplete right bundle branch block, and right ventricular hypertrophy. The findings were congruent with significant structural cardiac pathology (Figure 1A). Subsequent echocardiographic evaluation showed a moderate-sized mid-muscular ventricular septal defect with left-to-right shunt and moderate-to-severe pulmonary valve stenosis, with a peak systolic gradient of 60 mmHg across the pulmonary valve. These findings prompted further diagnostic work-up to delineate the anatomical details and assess the physiological impact.
FURTHER DIAGNOSTIC WORK-UP:
A detailed transthoracic echocardiogram (TTE) was performed, which confirmed the presence of a mid-muscular ventricular septal defect (VSD), measuring approximately 1.2 cm with a notable left-to-right shunt, and severe obstruction at the pulmonary valve with evidence of right ventricular hypertrophy (RVH). Notably, there was no indication of perimembranous VSD involvement. Additionally, the TTE findings raised concerns for potential left ventricular enlargement due to volume overload, necessitating further evaluation.
Chest radiography revealed signs consistent with pulmonary over-circulation and a question of left ventricular enlargement, suggesting volume overload secondary to the VSD. These imaging findings, alongside clinical symptoms of dyspnea on exertion, were indicative of hemodynamically significant shunting and pulmonary blood flow obstruction. Comparative chest radio-graphs taken 10 years apart provided visual evidence of the cardiac and pulmonary changes over time, as presented in Figure 2.
Cardiac catheterization corroborated these observations, demonstrating a moderate pressure gradient across the pulmonary valve before the intervention, which substantiated the decision to perform surgery. Hemodynamic measurements revealed pulmonary artery pressures that were reflective of a significant left-to-right shunt due to the VSD, confirming the need for closure of the defect to prevent further cardiac compromise.
DECISION FOR INTERVENTION:
The convergence of clinical presentation, diagnostic findings, and hemodynamic data informed a multidisciplinary team’s decision to proceed with concurrent transcatheter interventions. The significant RVH noted on echocardiography and ECG, in the context of symptomatic exercise intolerance, emphasized the urgent need for corrective measures.
INTERVENTIONAL PROCEDURE:
Under general anesthesia, the patient underwent a transcatheter closure of the muscular VSD using a number 6 Amplatzer device. The procedure was meticulously carried out with precise positioning of the device, ensuring complete coverage of the defect. The precise placement of the VSD closure device, as visualized in Figure 3A, ensured optimal closure of the defect without impinging on surrounding cardiac structures. Subsequently, the pulmonary valve was addressed through a balloon valvuloplasty approach, successfully dilating the valve and reducing the transvalvular pressure gradient. The balloon valvuloplasty procedure is shown in Figure 4A, where the mid-balloon waist impression indicates the stenotic region of the pulmonary valve before full dilation. After the successful dilatation of the pulmonary valve using balloon valvuloplasty, a marked decrease in the waist impression of the balloon was observed, indicative of valve opening, and the measured pressure gradient across the valve was significantly reduced, confirming the immediate efficacy of the intervention. Figure 4B shows the fully inflated balloon across the pulmonary valve after dilatation, with no waist impression, indicative of successful valvular opening and decreased pressure gradient. To further illustrate the postoperative structural outcomes, an intraoperative transesophageal echocardiogram (TEE) was performed, displaying the closure device in situ, as depicted in Figure 5. Immediate postoperative echocardiography confirmed the absence of residual shunting across the VSD and a remarkable reduction in the pulmonary valve gradient.
POSTOPERATIVE FOLLOW-UP AND OUTCOME:
The patient tolerated the procedure well and his postoperative course was marked by a remarkable improvement in symptoms. This once tachypneic individual now displayed stable respiratory rates and an absence of dyspnea on exertion. Follow-up assessments showed normalization of the right ventricular size and function, with echocardiograms depicting a stable VSD device position without residual shunts. Importantly, the previously noted right ventricular hypertrophy on ECG had notably regressed, indicating a successful alleviation of the pressure overload. Post-intervention electrocardiographic evaluation revealed a return to normal sinus rhythm and resolution of right ventricular hypertrophy, as demonstrated in Figure 1B, reflecting the successful reduction of cardiac workload following the transcatheter procedures. The post-intervention cardiac catheterization hemodynamics mirrored these positive developments, showing normalized pulmonary artery pressures and a trivial pulmonary valve gradient, consistent with a resolved pulmonary stenosis. Table 1 outlines the key pre- and post-intervention hemodynamic measurements obtained via right heart catheterization.
Postoperatively, the patient reported a marked improvement in exercise tolerance and a resolution of the atypical chest pain. Follow-up echocardiography confirmed the stability of the device, with a mild residual leak and normal left ventricular size and function. Figure 3B provides a detailed echocardiographic view of the well-positioned VSD closure device after the intervention, with no residual shunting. The ejection fraction was maintained at 55%, with no evidence of ventricular dilatation. The trace tricuspid regurgitation noted was not hemodynamically significant, and the right ventricular systolic pressure (RVSP) was estimated at 25–30 mmHg, suggesting the absence of severe pulmonary hypertension. The echocardio-graphic assessment provided crucial insights into the cardiac structures and function both before and after the transcatheter intervention. These findings are summarized in Table 2 below.
The integrated approach to management, involving both the pulmonic valvuloplasty and VSD closure, was instrumental in alleviating the patient’s symptoms and improving cardiac function. These findings highlight the necessity of individualized treatment strategies and the utility of transcatheter techniques in complex CHD cases.
An exercise stress test was negative for ischemia and arrhythmia at the achieved workload. The patient was subsequently discharged. This case exemplifies the effectiveness of combining transcatheter VSD closure and pulmonary valvuloplasty in treating complex congenital heart defects. The patient’s recovery trajectory highlights the potential of these less invasive modalities to yield substantial symptomatic relief and physiological correction, presenting a viable alternative to traditional surgical approaches.
Discussion
This case demonstrates the effective use of simultaneous trans-catheter closure of VSD and pulmonary valvuloplasty in an adult patient with coexisting defects. While combined trans-catheter procedures have been documented, reports have focused largely on pediatric populations [11,12]. The application in an adult patient is unique and provides valuable additions to the literature supporting this approach.
The decision to opt for transcatheter intervention over conventional surgery in this instance was multifaceted. Catheter-based techniques have demonstrated reduced morbidity and mortality compared to surgical alternatives [10]. Balloon valvuloplasty is the preferred first-line treatment for pulmonary stenosis, showing superior outcomes compared to surgical valvotomy [5,6]. Additionally, the Amplatzer device is renowned for its high VSD closure success rates and minimal complications, further justifying its application in this case [7,13,14].
This case exemplifies the advancements in medical technology that have increased the popularity and success of transcatheter interventions. Our report corroborates the findings of previous studies, showing the effectiveness, safety, and lower complication rates associated with balloon valvuloplasty [12,15] and the Amplatzer device for VSD closure [7,13,14,16,17]. The alignment of our findings with the existing literature reinforces the value of these less invasive modalities in managing intricate cases like the one presented here.
Employing both pulmonary balloon valvuloplasty and device closure of VSD in a single transcatheter session underscores the versatility and efficiency of this approach [18]. This combined strategy minimizes the need for multiple interventions, thereby reducing the patient’s exposure to procedural risks and improving recovery time [19]. Furthermore, the successful outcome of this combined approach in the present adult patient shows its potential applicability across diverse age groups and varied anatomical complexities [20,21]. The integration of these techniques into a single session can streamline clinical workflows, optimize resource utilization, and potentially lead to better patient experiences and outcomes [22]. Our report accentuates the need for further studies to validate the broader clinical implications and establish standardized protocols for such combined transcatheter interventions [23].
However, the insights drawn from this case study are not without limitations. The single-case design inherently restricts the generalizability of the results, and the absence of long-term follow-up impedes the assessment of the interventions’ durability. To address these limitations and to comprehensively evaluate the long-term impacts and efficacy of such treatments, further research through larger multi-center studies and randomized trials is needed. This case report serves as a foundational reference, calling for extended research to validate the effectiveness of these interventions in diverse patient demographics and to ascertain their long-term outcomes.
In summary, this report emphasizes the potential of concurrent transcatheter interventions in managing patients with coexisting VSD and severe valvar pulmonic stenosis. It corroborates and builds upon previous studies [11,12,24,25], reinforcing the safety and efficacy of these techniques and highlighting the need for continued research to further explore their applicability and benefits. The success of the treatment in this adult patient sheds light on the broader possibilities of transcatheter interventions, paving the way for future investigations aimed at refining and expanding these treatment options for congenital heart diseases.
Conclusions
In summary, our case reaffirms the viability of simultaneous transcatheter closure of ventricular septal defects and balloon valvuloplasty as a promising therapeutic approach for patients diagnosed with ventricular septal defects and severe valvar pulmonic stenosis. Our treatment of a 19-year-old male patient effectively demonstrates the safe and efficacious implementation of this strategy. These findings align with prior research and contribute to the body of literature endorsing transcatheter interventions for managing congenital heart diseases. Further investigations are warranted to ascertain the enduring effectiveness of this procedure and establish its superiority over surgical alternatives. These interventions use a less invasive method, yielding lower morbidity and mortality rates, and thus potentially offering improved patient outcomes.
Figures
References:
1.. Sun R, Liu M, Lu L, Congenital heart disease: Causes, diagnosis, symptoms, and treatments: Cell Biochem Biophys, 2015; 72(3); 857-60
2.. Lopez L, Houyel L, Colan SD, Classification of ventricular septal defects for the eleventh iteration of the international classification of diseases-striving for consensus: A report from the International Society for Nomenclature of Paediatric and Congenital Heart Disease: Ann Thorac Surg, 2018; 106(5); 1578-89
3.. Judge P, Meckler Mshs G, Congenital heart disease in pediatric patients: Recognizing the undiagnosed and managing complications in the Emergency Department: Pediatr Emerg Med Pract, 2016; 13(5); 1-28 ; quiz 27–28
4.. Bouhlel I, Ajmi H, Slim M, Immediate results of balloon valvuloplasty in congenital pulmonary valve stenosis: Tunis Med, 2021; 99(2); 291-97
5.. Rao PS, Balloon pulmonary valvuloplasty: A review: Clin Cardiol, 1989; 12(2); 55-74
6.. Bian C, Ma J, Wang J, Perimembranous ventricular septal defect with aneurysm: Two options for transcatheter closure: Tex Heart Inst J, 2011; 38(5); 528-32
7.. Chungsomprasong P, Durongpisitkul K, Vijarnsorn C, The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil: Catheter Cardiovasc Interv, 2011; 78(7); 1032-40
8.. Kenny D, Interventional cardiology for congenital heart disease: Korean Circ J, 2018; 48(5); 350-64
9.. Schmaltz AA, Bein G, Grävinghoff L, Balloon valvuloplasty of pulmonary stenosis in infants and children – co-operative study of the German Society of Pediatric Cardiology: Eur Heart J, 1989; 10(11); 967-71
10.. McElhinney DB, Quartermain MD, Kenny D, Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: A case-control study: Circulation, 2016; 133(18); 1738-46
11.. Xu XD, Bai Y, Chen XL, Simultaneous transcatheter treatment of perimembranous ventricular septal defect and other congenital cardiopathies: Heart Lung Circ, 2014; 23(12); 1169-74
12.. Ardakani MB, Haddadzadeh M, Rajaei S, Simultaneous transcatheter closure of ventricular septal defect and pulmonary valvuloplasty: Iran J Pediatr, 2012; 22(4); 555-58
13.. Brown KN, Adnan G, Kanmanthareddy A, Catheter management of ventricular septal defect: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing [cited 2023 Oct 30]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK538177/
14.. Du ZD, Hijazi ZM, Kleinman CS, Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial: J Am Coll Cardiol, 2002; 39(11); 1836-44
15.. Alsoufi B, Management of the single ventricle and potentially obstructive systemic ventricular outflow tract: J Saudi Heart Assoc, 2013; 25(3); 191-202
16.. El-Kadeem S, El Nemr S, El Amrousy D, Zoair A, Comparison of transcatheter versus surgical closure of perimembranous ventricular septal defect in pediatric patients: A systematic review and meta-analysis: J Saudi Heart Assoc, 2019; 31(4); 188-97
17.. Yi K, You T, Ding Z, Comparison of transcatheter closure, mini-invasive closure, and open-heart surgical repair for treatment of perimembranous ventricular septal defects in children: Medicine (Baltimore), 2018; 97(40); e12583
18.. Davidson LJ, Davidson CJ, Transcatheter treatment of valvular heart disease: A review: JAMA, 2021; 325(24); 2480-94
19.. Stout KK, Daniels CJ, Aboulhosn JA, 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: J Am Coll Cardiol, 2019; 73(12); 1494-563
20.. Butera G, Carminati M, Chessa M, Transcatheter closure of perimembranous ventricular septal defects: Early and long-term results: J Am Coll Cardiol, 2007; 50(12); 1189-95
21.. Butera G, Carminati M, Chessa M, Percutaneous versus surgical closure of secundum atrial septal defect: Comparison of early results and complications: Am Heart J, 2006; 151(1); 228-34
22.. Wilson W, Taubert KA, Gewitz M, A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group: Circulation, 2007; 116(15); 1736-54
23.. Vahanian A, Alfieri O, Andreotti F, Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS): Eur J Cardiothorac Surg, 2012; 42(4); S1-44
24.. Asada D, Tomita H, Fujii T, Successful simultaneous transcatheter treatment for a secundum atrial septal defect complicated by valvular pulmonary stenosis in an infant: Cardiol Young, 2018; 28(10); 1162-64
25.. Lu X, Wen P, Liu Y, Simultaneous percutaneous interventional treatment of atrial septal defects and pulmonary valve stenosis in children under the guidance of transoesophageal echocardiography alone: Preliminary experiences: Front Cardiovasc Med, 2022; 9; 772181
Figures
Tables
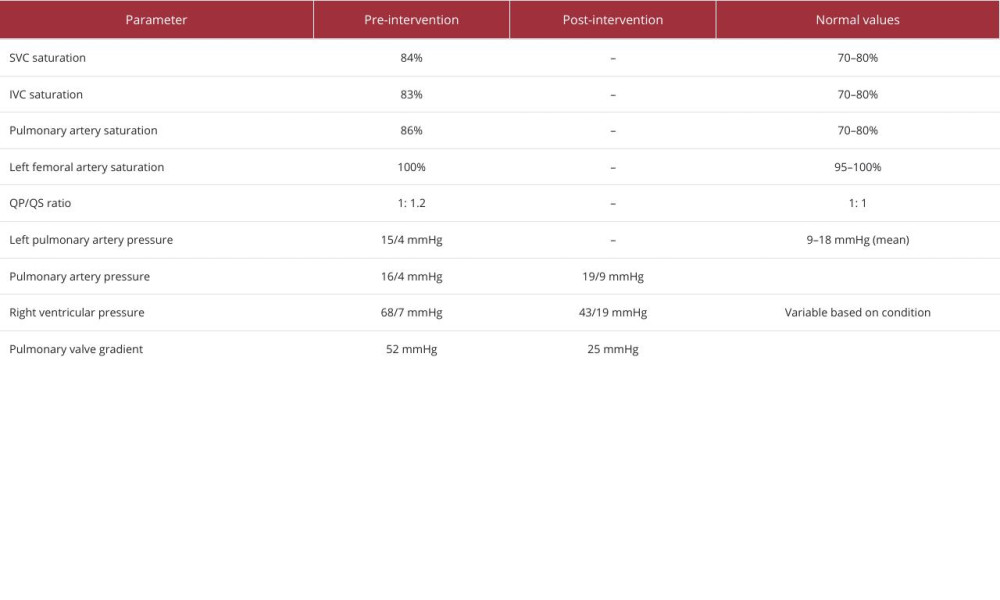 Table 1.. Hemodynamic parameters before and after transcatheter intervention.
Table 1.. Hemodynamic parameters before and after transcatheter intervention.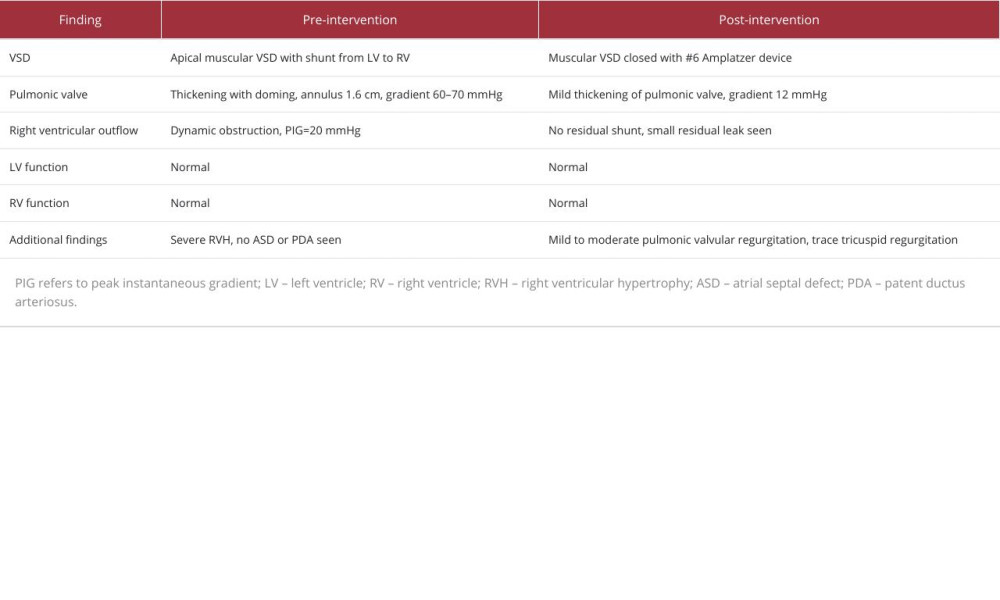 Table 2.. Pre- and post-intervention echocardiographic assessment of cardiac structures and function.
Table 2.. Pre- and post-intervention echocardiographic assessment of cardiac structures and function. Table 1.. Hemodynamic parameters before and after transcatheter intervention.
Table 1.. Hemodynamic parameters before and after transcatheter intervention. Table 2.. Pre- and post-intervention echocardiographic assessment of cardiac structures and function.
Table 2.. Pre- and post-intervention echocardiographic assessment of cardiac structures and function. In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








