26 January 2024: Articles 
Rapid and Effective Treatment of Peritonitis in Peritoneal Dialysis Patients with Intravenous Dalbavancin
Unusual or unexpected effect of treatment
Julia I. Tegethoff1DE, Isaac Teitelbaum2ABDF, Tyree H. Kiser1ABCDEF*DOI: 10.12659/AJCR.942755
Am J Case Rep 2024; 25:e942755
Abstract
BACKGROUND: Peritonitis is a complication associated with peritoneal dialysis (PD), which carries a significant morbidity and mortality risk. Empiric therapy must include coverage of gram-positive organisms; vancomycin is a recommended treatment option, particularly when MRSA infection is a risk. Vancomycin is cumbersome for patients, requiring therapeutic drug monitoring and re-administration by a healthcare provider. Dalbavancin, administered as a one-time intravenous dose, is a convenient potential treatment option for PD patients to cover gram-positive organisms without the need for routine drug monitoring.
CASE REPORT: We present 2 patients effectively treated with dalbavancin for infectious peritonitis. The first patient, a 73-year-old woman with end-stage renal disease (ESRD) on PD, presented to the hospital with fever, elevated white blood cells (WBCs), and cloudy peritoneal fluid with elevated nucleated cell counts (88% neutrophils). This patient was given 1 dose of 1500 mg IV dalbavancin. Within 3 days, her fever resolved, WBCs returned to normal, and peritoneal fluid results improved. The second patient was a 36-year-old woman presenting to an outpatient clinic with abdominal pain and cloudy peritoneal fluid with elevated nucleated cell counts (53% neutrophils) treated with dalbavancin 1500 mg IV once. Within 4 days, this patient’s pain had resolved, and peritoneal fluid results returned to baseline. No adverse effects were noted for either patient.
CONCLUSIONS: These cases illustrate the potential of dalbavancin as a convenient option for patients with PD-associated peritonitis. Both patients demonstrated rapid and complete response to a single dose of dalbavancin without complications. Further prospective studies are needed to establish dalbavancin as an option for peritonitis.
Keywords: Dalbavancin, Peritoneal Dialysis, peritonitis
Background
In 2021, 750 000 patients were living with end-stage renal disease (ESRD) in the United States. It is estimated that 110 000–115 000 new cases of ESRD are diagnosed and the patients begin renal replacement therapy each year, with 10% of these patients using peritoneal dialysis (PD) [1]. The use of home dialysis has increased significantly (35% increase) in the last decade, with 95% of home dialysis patients undergoing PD [2]. Infectious peritonitis is a serious complication associated with PD and accounts for about 18% of infection-related mortality in PD patients. Severe cases can lead to sepsis, loss of ultrafiltration, and peritoneal membrane failure, which can result in technical failure and default to traditional hemodialysis methods. Because of this, it is essential to rapidly and effectively treat peritonitis to resolve inflammation and preserve peritoneal membrane function [2]. Most PD-associated peritonitis cases are caused by gram-positive organisms, estimated at 45–65% of all cases [3,4]. As such, antimicrobial therapy is the predominant treatment for PD-associated peritonitis and should cover the common gram-positive pathogens associated with peritonitis, including
Vancomycin, a glycopeptide antibiotic, is effective in peritonitis for empiric gram-positive coverage but requires frequent drug concentration monitoring to assess drug efficacy. Additionally, it must be re-administered every 3–5 days to maintain steady-state concentrations of 15–20 mcg/mL, which can be burdensome and unmanageable for patients. Dalbavancin, a synthetic lipoglycopeptide, has a similar mechanism of action to vancomycin, yet has shown improved potency against gram-positive organisms and longer half-life (approximately 1 week) when compared to older glycopeptide antibiotics [7]. One pharmaco-kinetic study demonstrated that throughout a 14-day period, a single 1500-mg IV dose of dalbavancin achieved sufficient plasma and peritoneal fluid concentrations necessary to treat peritonitis caused by gram-positive pathogens [8]. Dalbavancin offers a convenient and effective option for PD patients as a one-time dose without the need for routine plasma concentration monitoring. As a one-time dose, dalbavancin can be easily used in the outpatient and home-health settings rather than the inpatient setting due to the lack of needed repeated doses and monitoring. Here, we present 2 patients with PD-associated peritonitis effectively treated with empiric dalbavancin therapy.
Case Reports
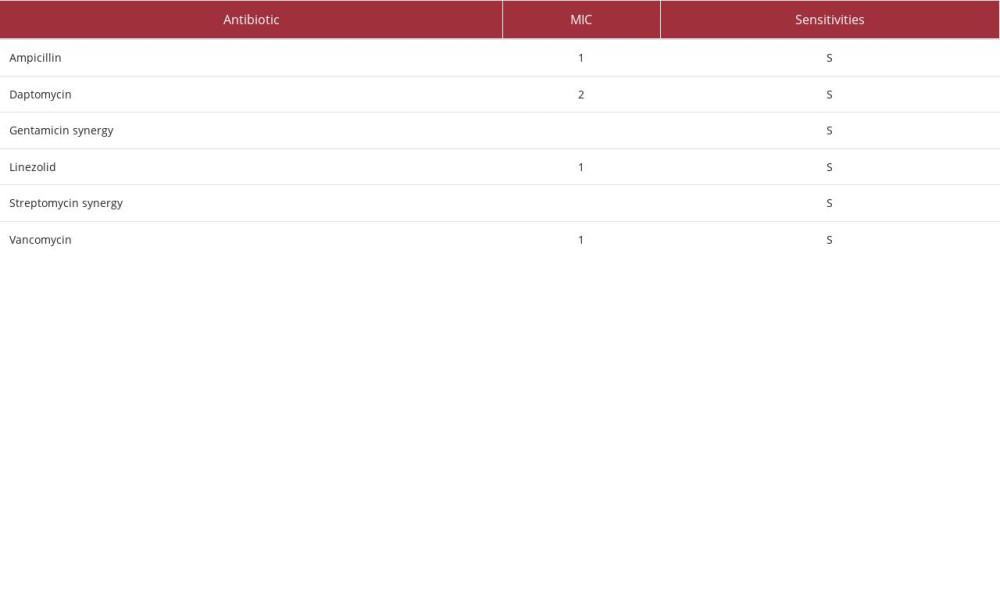
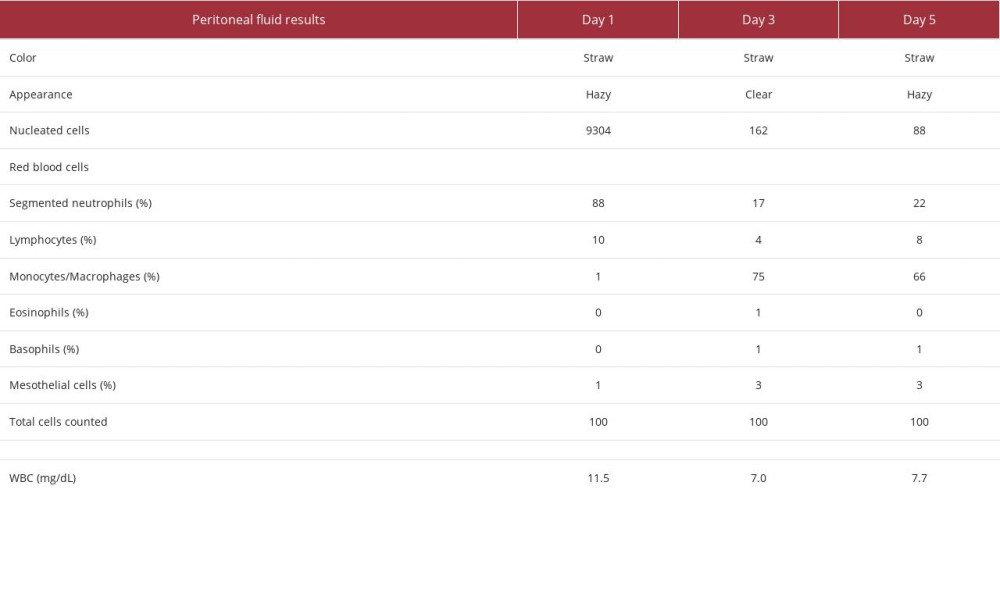
Our first patient was a 73-year-old woman who presented to the hospital with abdominal pain and fever of 36.1°C. Her medical history was significant for ESRD on home PD, hyper-tension, type 2 diabetes mellitus, congestive heart failure, and granulomatosis with polyangiitis. Her home PD prescription was for nocturnal intermittent peritoneal dialysis (NIPD) with 2.5% dextrose solution, fill volume of 2800 mL, total volume of 11 200 mL, and 9-h intervals. This patient was admitted on Day 1 with a serum creatinine of 6.56 mg/dL, white blood cell (WBC) count of 11.1 mg/dL, and a cloudy and straw-colored peritoneal fluid sample with 9304 nucleated cells, 88% of which were neutrophils. Peritoneal fluid was cultured and empiric antibiotics covering gram-positive, gram-negative, and fungal organisms were started. This patient was given a single dose of dalbavancin 1500 mg IV, ceftazidime 1 g IP daily, and fluconazole 100 mg IV daily. On Day 3, culture results and sensitivities were available (Table 1), showing
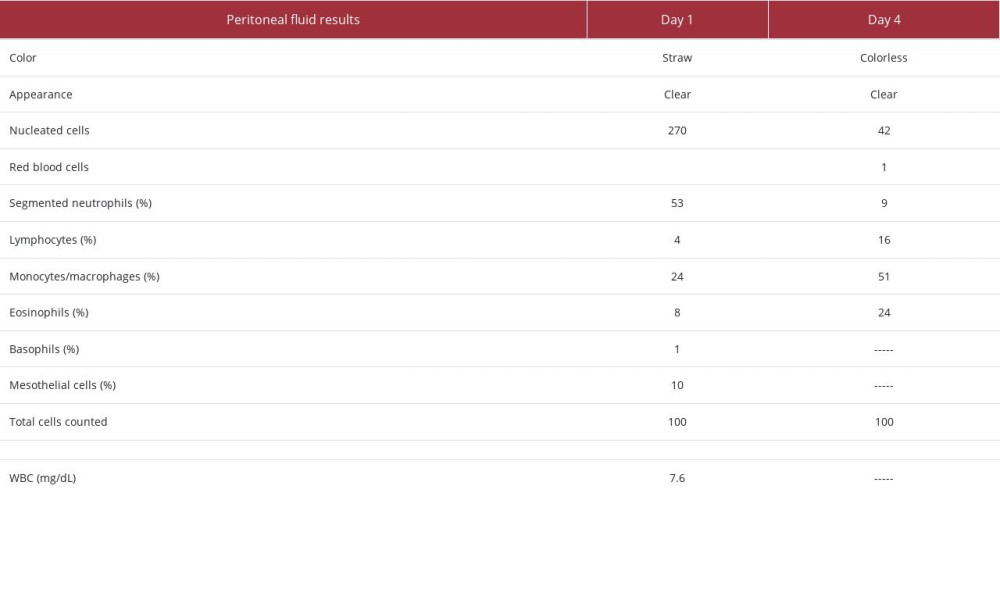
The second patient was a 36-year-old woman who presented to an outpatient clinic on Day 1 with abdominal pain. Her medical history was significant for ESRD on home PD, hyper-tension, type 2 diabetes mellitus, chronic glomerulonephritis, hypothyroidism, and obesity. Her home PD prescription was for continuous ambulatory peritoneal dialysis (CAPD) with icodextrin and 2.5% dextrose solution, fill volume of 2000 mL, total volume of 6000 mL, and 8-h intervals. Her serum creatinine was 4.61 mg/dL, temperature of 36.5°C, and WBC count of 7.6 mg/dL. Peritoneal fluid results (Table 3) showed straw-colored, clear fluid with 270 nucleated cells, 53% of which were neutrophils. Peritoneal fluid cultures were drawn, and the patient was started on empiric antibiotics covering gram-positive and gram-negative organisms with a single dose of dalbavancin 1500 mg IV and 1 dose of ceftazidime 1 g IP. Upon follow-up on Day 4, the patient reported resolution of abdominal pain and the peritoneal fluid was clear and colorless, with 42 nucleated cells, 9% of which were neutrophils. No organisms were present in peritoneal fluid culture. Throughout therapy, this patient reported no adverse effects or complications related to dalbavancin therapy.
Discussion
These cases illustrate the potential of dalbavancin as a therapeutic option for patients with PD-associated infectious peritonitis. Both patients demonstrated rapid and complete response to a single dose of dalbavancin without complications or adverse effects. Dalbavancin is currently FDA-approved to treat skin and soft-tissue infections only [9]. Uses beyond this are being investigated, including osteomyelitis, endocarditis, blood stream infections, pneumonia, and catheter-related infections [10–13]. Little data exists on treating intrabdominal infections, like peritonitis, with dalbavancin. However, the DALBACEN cohort study showed that a single dose of dalbavancin 1500 mg IV was effective to treat gram-positive blood stream infections, with only 4.8% of patients experiencing adverse effects (all grade 3 or lower) [10]. Additionally, previous pharmacokinetic studies have shown that a single 1500 mg IV dose of dalbavancin produces adequate, sustained peritoneal fluid concentrations required to treat peritonitis caused by gram-positive infections for at least 14 days [8]. From these data, we can infer that dalbavancin is effective in treating gram-positive infections and sufficiently distributes to perito-neal fluid to treat peritonitis caused by gram-positive organisms. Our case study supports this, as both of our patients had quick and sustained resolution of their peritonitis infections after dalbavancin therapy.
Using dalbavancin for these infections may relieve patients of many obstacles currently faced with vancomycin therapy, such as frequent hospital trips and healthcare-associated costs. Many patients with ESRD choose PD over traditional hemo-dialysis for the benefit of home care. If peritonitis occurs and patients undergo treatment with vancomycin, they will require frequent drug monitoring and re-administration every 3–5 days for up to 14 days. This may result in repeat visits to inpatient or specialized outpatient care centers or multiple specialized home-health visits. Alternatively, dalbavancin is a single dose and does not require therapeutic drug monitoring, so patients would visit healthcare facilities less often and utilize fewer resources, avoiding many costs. Although the per-vial cost of dalbavancin is more than vancomycin, the costs saved avoiding labs and frequent visits for up to 14 days of vancomycin therapy can save patients substantial amounts [14]. This was observed in our case study, particularly with Patient 2, who was treated in an outpatient clinic and was required to return to the clinic on Day 4 only to provide a peritoneal fluid sample. However, it is important to note that decreasing frequent monitoring and drug administration with a one-time dose in the inpatient setting, as observed with Patient 1, also underlines the economic utility of dalbavancin. Dalbavancin proved to be effective in treating both patients’ peritonitis and minimized healthcare-associated costs. Both patients saw a complete resolution of PD-associated peritonitis symptoms without recrudescence.
Limitations of this report include the small number of patients who received dalbavancin. Further studies using dalbavancin to treat peritonitis can provide more information on this treatment approach. Dalbavancin is a suitable alternative to vancomycin in the treatment of PD-associated peritonitis, but it does not overcome some of the challenges seen with vancomycin, such as in culture-positive peritonitis caused by non-gram-positive pathogens. Although most PD-associated peritonitis cases are gram-positive, if the causative pathogen is not, dalbavancin’s use could unnecessarily expose the patient to a broad-spectrum agent [4]. Additionally, dalbavancin’s utility is limited by type and susceptibility of the causative organism. Dalbavancin’s place in therapy is likely not as an empiric treatment, but rather as a definitive single-dose option chosen once it is confirmed that the causative organism is gram-positive and empiric therapy is narrowed. In the case of resistance to dalbavancin upon availability of sensitivities, if the organism is not susceptible to dalbavancin as evidenced by elevated MIC, an alternative gram-positive agent may be necessary.
Conclusions
We report the successful use of dalbavancin to treat PD-associated peritonitis. Dalbavancin is administered in a single dose versus its alternative, vancomycin, which requires therapeutic drug monitoring and re-administration requiring patients to return to healthcare facilities multiple times. This can be cumbersome for patients and may result in non-adherence, incomplete response, and high healthcare-associated costs. Further prospective clinical studies are needed to establish dalbavancin as a therapeutic option in peritonitis.
References:
1.. Johansen KL, Chertow GM, Gilbertson DT, US Renal Data System 2021 annual data report: epidemiology of kidney disease in the United States: Am J Kidney Dis, 2022; 79(4); A8-A12
2.. Li PK, Chow KM, Cho Y, ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment: Perit Dial Int, 2022; 42(2); 110-53
3.. Mujais S, Microbiology and outcomes of peritonitis in North America: Kidney Int Suppl, 2006(103); S55-62
4.. Oo TN, Roberts TL, Collins AJ, A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients: Am J Kidney Dis, 2005; 45(2); 372-80
5.. Li PK-T, Szeto CC, Piraino B, ISPD Peritonitis Recommendations: 2016 Update on prevention and treatment: Perit Dial Int, 2016; 36(5); 481-508
6.. Szeto C-C, Li PK-T, Johnson DW, ISPD catheter-related infection recommendations: 2017 update: Perit Dial Int, 2017; 37(2); 141-54
7.. Molina KC, Miller MA, Mueller SW, Clinical pharmacokinetics and pharmacodynamics of dalbavancin: Clin Pharmacokinet, 2022; 61(3); 363-74
8.. Van Matre ET TI, Kiser TH, Intravenous and intraperitoneal pharmacokinetics of dalbavancin in peritoneal dialysis patients: Antimicrob Agents Chemother, 2020; 64(5); e02089-19
9.. : Dalvance(R) package insert, 2016, Parsippany, NJ, Durata Therapeutics U.S. Limited
10.. Hidalgo-Tenorio C, Vinuesa D, Plata A, DALBACEN cohort: Dalbavancin as consolidation therapy in patients with endocarditis and/or bloodstream infection produced by gram-positive cocci: Ann Clin Microbiol Antimicrob, 2019; 18(1); 30
11.. Morrisette T, Miller MA, Montague BT, On- and off-label utilization of dalbavancin and oritavancin for Gram-positive infections: J Antimicrob Chemother, 2019; 74(8); 2405-16
12.. de Pablo-Miró M, Pujol-Ruiz S, Iftimie S, Comparative analysis of dalbavancin versus other antimicrobial options for gram-positive cocci infections: Effectiveness, hospital stay and mortality: Antibiotics, 2021; 10(11); 1296
13.. Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB, Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: A systematic review: Int J Antimicrob Agents, 2020; 56(3); 106069
14.. Andruszko B, Khadem T, Huntress J, Dobson E, Ashley ED, Pharmacoeconomic analysis to compare dalbavancin and vancomycin in an outpatient parenteral antimicrobial therapy (OPAT) population: Open Forum Infectious Diseases, 2015; 2; ofv133.1014
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250