18 August 2020: Articles 
Airway Hygiene in COVID-19 Pneumonia: Treatment Responses of 3 Critically Ill Cruise Ship Employees
Unusual clinical course, Unusual or unexpected effect of treatment, Educational Purpose (only if useful for a systematic review or synthesis)
Faryal I. Farooqi1ABCDEFG, Richard C. Morgan1ABCDEFG*, Naveen Dhawan1CDEF, John Dinh1ADEF, George Yatzkan2ABCE, George Michel1ABCEDOI: 10.12659/AJCR.926596
Am J Case Rep 2020; 21:e926596
Abstract
BACKGROUND: COVID-19, the disease entity caused by the novel severe acute respiratory coronavirus 2 (SARS-CoV-2), continues to pose a major therapeutic challenge for clinicians. At present, an effective treatment regimen and vaccination has not been established. Many patients develop severe symptoms requiring endotracheal intubation and a prolonged stay in the Intensive Care Unit (ICU). In early postmortem examinations of COVID-19 patients, profuse viscous secretions were observed throughout the respiratory tract. Thus, oxygen supplementation without aggressive pulmonary hygiene management may be suboptimal. In the present case series, pulmonary hygiene management encompassed mucolytics, bronchodilators, and tracheal suctioning. We report 3 severe cases of COVID-19 pneumonia in cruise ship employees who were admitted to the ICU and responded to supportive mechanical ventilation and pulmonary hygiene management.
CASE REPORT: Three cruise ship employees with COVID-19 underwent endotracheal intubation and were admitted to the ICU for acute hypoxemic respiratory failure. Initial chest X-rays suggested multifocal pneumonia with superimposed acute respiratory distress syndrome (ARDS). A regimen of hydroxychloroquine, azithromycin, and dexamethasone was initiated on admission in all cases. Additionally, medications used for pulmonary hygiene were administered through a metered-dose inhaler (MDI) in line with the ventilator circuit. Endotracheal suctioning was performed prior to medication administration. The duration from endotracheal intubation to extubation ranged from 9 to 24 days. All 3 patients reached 30-day survival.
CONCLUSIONS: The cases reported highlight the importance of the use of airway hygiene with mucolytics, bronchodilators, and tracheal suctioning for patients with COVID-19 pneumonia requiring ventilatory support.
Keywords: Bronchodilator Agents, COVID-19, Critical Care, Expectorants, Respiratory Distress Syndrome, Adult, Respiratory Therapy, Antimalarials, Betacoronavirus, COVID-19, Coronavirus Infections, Critical Illness, Disease Transmission, Infectious, hydroxychloroquine, Hygiene, Pandemics, Pneumonia, Viral, Respiration, Artificial, SARS-CoV-2, Ships
Background
In December 2019 the novel respiratory illness SARS-COV-2 broke out in Wuhan, China and rapidly spread across the globe, giving rise to the current COVID-19 pandemic. As of July 15, 2020, the World Health Organization (WHO) reported there were 13 150 645 confirmed cases throughout the world [1]. Although SARS-COV-2 largely manifests mild or nonexistent symptoms, rapid clinical deterioration can arise within the initial 2 weeks of illness [2]. Severe respiratory symptoms are associated with atypical pneumonia that can progress to acute respiratory distress syndrome (ARDS), although the pathogenesis is still being elucidated. COVID-19-related acute respiratory distress syndrome (ARDS) has been described and usually results in acute hypoxemic respiratory failure (AHRF) [3]. The incidence of COVID-19-related ARDS has ranged from 3.4% to 31% in hospitalized patients [4–8]. Timely endotracheal intubation and transfer to the ICU is critical in these cases. In a study of risk factors for COVID-19 mortality, ARDS was found to occur in 93% of patients who did not survive [8]. Interestingly, COVID-19-related ARDS does not directly resemble the “typical” ARDS due to other causes [3,9]. Therefore, treatment strategies for typical ARDS may not be as effective in COVID-19-related ARDS. The unique disease traits of COVID-19 pose unprecedented healthcare challenges, requiring strong research efforts and innovative drug repurposing strategies.
In the absence of a specific antiviral medication and vaccination, the tenets of current management have been preventive and supportive care. For cases of AHRF, high-flow nasal oxygen (HFNO) therapy can treat hypoxia and can avert the need for supportive mechanical ventilation [10]. Titration of HFNO to a target oxygen saturation (SpO2) of 90–96% along with a low threshold for endotracheal intubation has been advocated [11]. Recently, a multicenter study on COVID-19 outcomes in the New York City area reported that 320 of 2534 (12.2%) patients who were discharged alive or who died underwent supportive mechanical ventilation during their hospital course [12]. The mainstay of supportive mechanical ventilation in COVID-19 patients with AHRF is protective lung ventilation, which consists of a low tidal volume (≤6 mL/kg predicted body weight) and plateau pressure ≤30 cm H2O [11,13]. Patients with refractory hypoxemia may be considered for prone positioning, higher levels of positive end-expiratory pressure (PEEP), intravenous neuromuscular blocking agents, and extracorporeal membrane oxygenation (ECMO) [14]. Additionally, dexamethasone and high-dose vitamin C may help attenuate the aberrant inflammatory response in COVID-19-related ARDS [15,16]. A combination of treatments is needed to improve oxygenation and prevent clinical deterioration in critically ill patients with COVID-19.
An impressive distinction of COVID-19 pneumonia is the wide variability in disease progression and response to treatment. Gattinoni et al. suggested different phenotypes (Type-L and Type-H) according to lung elastance, ventilation to perfusion ratio, weight, and alveolar recruitability [17]. This corresponds with the amount of inflammatory response and consequent interstitial edema during pulmonary infection. Interestingly, postmortem studies on patients with a confirmed COVID-19 diagnosis have revealed extensive pulmonary mucous secretions and plugging [18,19]. Liu et al. described thick and sticky secretions in the trachea, bronchus, and alveoli in an autopsy with COVID-19 confirmed as the cause of death [20]. In accordance with these observations, stringent pulmonary hygiene management may be needed to optimize oxygen supplementation.
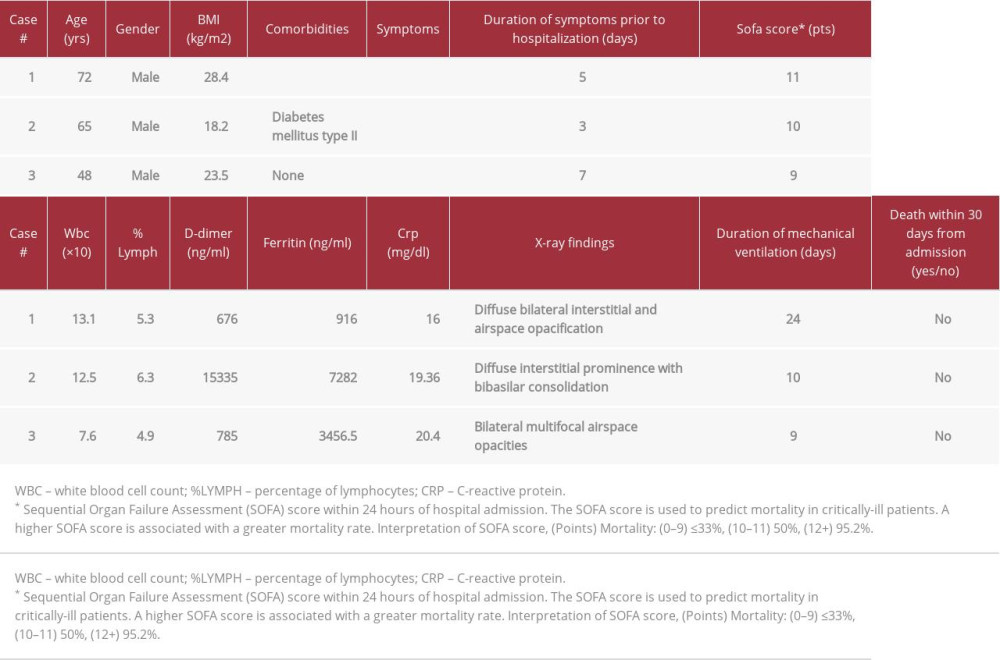
To the best of our knowledge, the role of pulmonary hygiene management in critically ill patients with SARS-COV-2 pneumonia has been largely overlooked in recent research. For the purpose of this case series, pulmonary hygiene management refers to mucolytics, bronchodilators, and tracheal suctioning. The utility of mucolytics, bronchodilators, and tracheal suctioning on the prevention of hospital-acquired pulmonary infections and mortality in the Intensive Care Unit (ICU) has been well studied in the past [21]. With the exception of bromhexine hydrochloride, a well-known mucolytic cough suppressant, research on modalities that improve mucus clearance in COVID-19 pneumonia is limited. The current report describes 3 cases of COVID-19 pneumonia, confirmed by reverse transcription-polymerase chain reaction (RT-PCR) testing from nasopharyngeal swabs, in critically ill cruise ship employees who developed ARDS and required supportive mechanical ventilation (Table 1). In this article, we explore the role of a therapeutic strategy consisting of inhaled albuterol, inhaled ipratropium, inhaled n-acetylcysteine, and routine tracheal suctioning in COVID-19 pneumonia and ARDS.
Case Reports
CASE 1:
A 72-year-old male cruise ship employee with a past medical history of coronary artery disease, chronic kidney disease, diabetes mellitus type II, and hypertension was brought to the Emergency Department (ED) with 5 days of worsening shortness of breath, dry cough and fever. In the 2 days prior to hospital admission, the patient received amoxicillin-clavulanic acid, azithromycin, and oseltamivir on the cruise ship, without improvement. At the time of hospital admission, the patient was dyspneic with an oxygen saturation (SpO2) of 82% on room air. ABG values revealed pH 7.37, PaO2 46.2, PCO2 29.3, HCO3 16.4, and SO2 83 on a nasal cannula. The initial chest X-ray (CXR) is illustrated in Figure 1A. A nasopharyngeal specimen was collected in the hospital and was confirmed to be COVID-19-positive by our laboratory. Blood lab results demonstrated an elevated white blood count (13.10) and neutrophil percentage (91.2%) along with decreased lymphocyte percentage (5.3%) in the Emergency Department. For the most part, vitals were within normal limits with the exception of respiratory rate; blood pressure (BP) 136/68 mmHg, pulse (HR) 95 beats per min respiratory rate (RR) 25 breaths per min temperature (TEMP) 37.8°C. The patient was put on a non-rebreather mask but continued to be restless and had difficulty forming full sentences. Despite initiation of non-invasive ventilation, he did not surpass 88% SpO2. Consequently, the patient underwent endotracheal intubation and a nasogastric tube was placed. Initial ventilator settings in the ICU were tidal volume (TV) 500 mL per inspiration, fraction of inspired oxygen (FiO2) 50%, respiratory rate (RR) 12 breaths per min (bpm), and PEEP 5 titrated to 8 cmH20. We administered subcutaneous heparin 5000 U every 8 h, oral azithromycin 250 mg every day, intravenous dexamethasone 12 mg every 6 h, oral hydroxychloroquine 400 mg every 12 h, oral vitamin C 1000 mg every day, and oral guaifenesin 600 mg every 6 h. During inhalation therapy, a closed circuit was maintained by depositing the metered-dose inhaler aerosol through a spacer device connected to the ventilator. Breathing treatments consisted of inhaled levalbuterol 1.25 mg every 6 h, inhaled ipratropium 0.5 mg every 6 h, and inhaled n-acetylcysteine 200 mg every 6 h. Prior to breathing treatments, tracheal suctioning was performed to remove secretions. Throughout his hospital course, the patient encountered numerous complications. He developed septic shock resulting in acute kidney failure and severe hypotension, which required emergent dialysis and titrated norepinephrine bitartrate. Additionally, multiple transfusions of packed red blood cells were administered for an acute gastrointestinal hemorrhage. These conditions improved by following the Kidney Disease Improving Global Outcomes (KDIGO), American College of Gastroenterology (ACG), and Surviving Sepsis Campaign clinical practice guidelines. The CXR completed on day 14 of treatment is shown in Figure 1B. On day 24 of hospitalization, the patient was extubated. At that time, he was downgraded from ICU status and remained on a venturi mask with oxygen saturation of 94–92%. At day 30 of hospitalization, he was still alive.
CASE 2:
A 65-year-old male cruise ship employee with a past medical history of diabetes mellitus type II presented to the ED with 3 days of chest tightness, shortness of breath, fever, and diarrhea. Upon arrival to the ED, the patient was severely dyspneic and unable to form full sentences. A non-rebreather mask was immediately placed because he was displaying these signs of respiratory distress. ABG values revealed pH 7.46, PaO2 57, PaCO2 35, HCO3 24, and SO2 91.1 on a non-rebreather mask. Vitals were within normal limits with the exception of respiratory rate; BP 123/65 mmHg, HR 94 bpm, RR 28 bpm, and TEMP 36.8°C. The initial chest X-ray (CXR) is shown in Figure 2A. Laboratory results from blood drawn on admission demonstrated an elevated white blood cell count (14.3) and neutro-phil percentage (84.1%), as well as a decreased lymphocyte percentage (6.3%). A nasopharyngeal specimen was collected and confirmed to be COVID-19-positive by the hospital laboratory. Although a non-rebreather mask was placed, he continued to be restless and dyspneic, and his SpO2 decreased to 88%. The patient was transferred to the ICU and underwent endotracheal intubation as well as placement of a nasogastric tube. Initial ventilator settings in the ICU were TV 500 mL per inspiration, RR 12 bpm, fraction of inspired oxygen (FiO2) 70%, and PEEP 6 cmH20. He received subcutaneous heparin 5000 U every 8 h, oral azithromycin 250 mg every day, intravenous dexamethasone 12 mg every 6 h, oral hydroxychloroquine 400 mg every 12 h, oral vitamin C 1000 mg every day, and oral guaifenesin 600 mg every 6 h. Additionally, we administered inhaled levalbuterol 1.25 mg every 6 h, inhaled ipratropium 0.5 mg every 6 h, and inhaled n-acetylcysteine 200 mg every 6 h. Tracheal suctioning was performed routinely, prior to medication administration. On day 1 in the ICU, the patient became hemodynamically unstable, requiring 3 days of titrated norepinephrine bitartrate. Over the following week, he remained hemodynamically stable and his respiratory status improved. On day 10, the patient was successfully extubated and started on non-invasive positive-pressure ventilation (NIPPV). The patient was comfortable and breathing well on the NIPPV and was weaned off supplemental oxygen. On room air, his SpO2 was 93.1% (day 11). Figure 2B illustrates his CXR on day 14 of treatment. Throughout the remainder of his hospital course, the patient sparingly used the nasal cannula provided. His discharge was delayed until day 39 for reasons outside the control of the physician and hospital.
CASE 3:
A 48-year-old male cruise ship employee without any relevant past medical history was brought to the ED with 7 days of worsening fever, dry cough, and shortness of breath. Upon arrival to the ED, he was anxious, restless, and dyspneic. ABG values revealed pH 7.51, PaO2 42.8, PaCO2 37.4, HCO3 29.6, and SO2 83 on a nasal cannula. The patient was immediately placed on a non-rebreather mask, which led to a marked improvement in SpO2 (93%) and his ability to breathe comfortably. In the ED, he had an elevated blood pressure, heart rate, and respiratory rate; BP 155/85 mmHg, HR 108, RR 28, TEMP 37.2°C. The initial CXR is shown in Figure 3A. Laboratory results from blood drawn in the ED demonstrated the white blood cell count was within normal limits (7.55), decreased lymphocyte percentage (8.9%), and increased neutrophil percentage (80.4%). The patient was admitted to the ICU on a non-rebreather mask. We administered subcutaneous heparin 5000 U every 8 h, oral azithromycin 250 mg every day, intravenous dexamethasone 12 mg every 6 h, oral hydroxychloroquine 400 mg every 12 h, oral vitamin C 1000 mg every day, and oral guaifenesin 600 mg every 6 h. In addition, he received inhaled levalbuterol 1.25 mg every 6 h, inhaled ipratropium 0.5 mg every 6 h, and inhaled n-acetylcysteine 200 mg every 6 h. Tracheal suctioning was performed prior to medication administration. The patient maintained the targeted SpO2 (95–93%) on a non-rebreather mask until day 5 of hospitalization. At that time, he abruptly desaturated to an SpO2 of 43% and emergent endotracheal intubation was immediately performed. Titrated norepinephrine bitartrate was started for hemodynamic instability. Ventilator settings were TV 500, FiO2 100%, RR 20, and PEEP 14. Shortly thereafter, his SpO2 improved to 93%. After 9 days of supportive mechanical ventilation, his respiratory status improved, and he was extubated. A CXR from hospital day 14 is illustrated in Figure 3B. Throughout the remainder of the hospital course, the patient remained on nasal cannula with SpO2 ranging between 98–93%. He was discharged from the hospital on day 41.
Discussion
LIMITATIONS:
Our findings were limited to 3 patients in a single community hospital intensive care unit (ICU). In all 3 cases, the patients were administered multiple medications according to popular treatment strategies at that time. Hydroxychloroquine, azithromycin, and dexamethasone have been proposed to improve outcomes in patients with COVID-19. At present, the efficacy of hydroxychloroquine and azithromycin is uncertain and has become a topic of fervent debate. Conversely, dexamethasone has been shown to improve mortality in severely ill patients with COVID-19 [15]. In addition, older age (65 years and above) and comorbid conditions is an indicator of poor prognosis in severe cases [53]. One of the patients mentioned in this case series was under the age of 65 years, which may have contributed to a better outcome.
Conclusions
This case series highlights the importance of pulmonary hygiene management in mechanically ventilated patients with COVID-19 pneumonia and ARDS. Our pulmonary hygiene strategy consists of mucolytics, bronchodilators, and routine tracheal suctioning. Inhaled medications were administered through a metered-dose inhaler (MDI) in line with a closed ventilator circuit. Implementation of this pulmonary hygiene strategy may prevent acute complications in the ICU as well as long-term sequelae after hospital discharge. All 3 critically ill patients in the present study were extubated, downgraded from ICU status, and reached 30-day survival.
Figures
References:
1.. : Coronavirus Disease (COVID-19) Situation Report, 2020
2.. Dreher M, Kersten A, Bickenbach J, The characteristics of 50 hospitalized COVID-19 patients with and without ARDS: Dtsch Arztebl Int, 2020; 117(16); 271-78
3.. Li X, Ma X, Acute respiratory failure in COVID-19: Is it “typical” ARDS?: Crit Care, 2020; 24(1); 198
4.. Chen N, Zhou M, Dong X, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study: Lancet, 2020; 395(10223); 507-13
5.. Guan WJ, Ni ZY, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
6.. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395(10223); 497-506
7.. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323(11); 1061-69
8.. Zhou F, Yu T, Du R, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study: Lancet, 2020; 395(10229); 1054-62
9.. Gattinoni L, Chiumello D, Rossi S, COVID-19 pneumonia: ARDS or not?: Crit Care, 2020; 24(1); 154
10.. Li J, Jing G, Scott JB, Year in review 2019: High-flow nasal cannula oxygen therapy for adult subjects: Respir Care, 2020; 65(4); 545-57
11.. Matthay MA, Aldrich JM, Gotts JE, Treatment for severe acute respiratory distress syndrome from COVID-19: Lancet Respir Med, 2020; 8(5); 433-34
12.. Richardson S, Hirsch JS, Narasimhan M, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area: JAMA, 2020; 323(20); 2052-59
13.. Aoyama H, Uchida K, Aoyama K, Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: A systematic review and network meta-analysis: JAMA Netw Open, 2019; 2(7); e198116
14.. : Coronavirus Disease 2019 (COVID-2019) Treatment Guidelines, 2020, National Institute of Health (NIH)
15.. Horby P, Lim WS, Emberson J, Effect of dexamethasone in hospitalized patients with COVID-19: Preliminary report. med: Rxiv; 2020; 20137273
16.. Fowler AA, Truwit JD, Hite RD, Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial: JAMA, 2019; 322(13); 1261-70
17.. Gattinoni L, Chiumello D, Caironi P, COVID-19 pneumonia: Different respiratory treatments for different phenotypes?: Intensive Care Med, 2020; 46(6); 1099-102
18.. Fox SE, Akmatbekov A, Harbert JL, Pulmonary and cardiac pathology in African American patients with COVID-19: A autopsy series from New Orleans: Lancet Respir Med, 2020; 8(7); 681-86
19.. Tang D, Comish P, Kang R, The hallmarks of COVID-19 disease: PLoS Pathog, 2020; 16(5); e1008536
20.. Qian L, Wang RS, Qu GQ, General observation report on system anatomy of dead corpses of new coronavirus pneumonia: Journal of Forensic Medicine, 2020; 36(1); 21-23
21.. Bassi GL, Airway secretions and suctioning: Principles and practice of mechanical ventilation, 2012, McGraw-Hill Education
22.. Goyal P, Choi JJ, Pinheiro LC, Clinical characteristics of Covid-19 in New York City: N Engl J Med, 2020; 382(24); 2372-74
23.. Ye Q, Wang B, Mao J, The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19: J Infect, 2020; 80(6); 607-13
24.. Nie S, Han S, Ouyang H, Zhang Z, Coronavirus Disease 2019-related dyspnea cases difficult to interpret using chest computed tomography: Respir Med, 2020; 167; 105951
25.. Barton LM, Duval EJ, Stroberg E, COVID-19 autopsies, Oklahoma, USA: Am J Clin Pathol, 2020; 153(6); 725-33
26.. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Targeting potential drivers of COVID-19: Neutrophil extracellular traps: J Exp Med, 2020; 217(6); e20200652
27.. Carsana L, Sonzogni A, Nasr A, Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study: Lancet Infect Dis, 2020 [Online ahead of print]
28.. Wang C, Xie J, Zhao L, Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients: EBioMedicine, 2020; 57; 102833
29.. Menter T, Haslbauer JD, Nienhold R, Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction: Histopathology, 2020 [Online ahead of print]
30.. Fahy JV, Dickey BF, Airway mucus function and dysfunction: N Engl J Med, 2010; 363(23); 2233-47
31.. Cole AM, Dewan P, Ganz T, Innate antimicrobial activity of nasal secretions: Infect Immun, 1999; 67(7); 3267-75
32.. Spagnolo P, Balestro E, Aliberti S, Pulmonary fibrosis secondary to COVID-19: A call to arms?: Lancet Respir Med, 2020; 8(8); 750-52
33.. Schaller T, Hirschbühl K, Burkhardt K, Postmortem examination of patients with COVID-19: JAMA, 2020; 323(24); 2518-20
34.. Antonio GE, Wong KT, Hui DSC, Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: Preliminary experience: Radiology, 2003; 228; 810-15
35.. Chan JF, Kok KH, Zhu Z, Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan: Emerg Microbes Infect, 2020; 9(1); 221-36
36.. Masoompour SM, Anushiravani A, Norouz AT, Evaluation of the effect of nebulized N-acetylcysteine on respiratory secretions in mechanically ventilated patients: Randomized clinical trial: Iran J Med Sci, 2015; 40(4); 309-15
37.. Jelic S, Cunningham JA, Factor P, Clinical review: Airway hygiene in the Intensive Care Unit: Crit Care, 2008; 12(2); 209
38.. Maggio R, Corsini GU, Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection: Pharmacol Res, 2020; 157; 104837
39.. Habtemariam S, Nabavi SF, Ghavami S, Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARSCoV-2 infection based on its action on the Transmembrane Serine Protease 2: Pharmacol Res, 2020; 157; 104853
40.. Grimes JM, Grimes KV, p38 MAPK inhibition: A promising therapeutic approach for COVID-19: J Mol Cell Cardiol, 2020; 144; 63-65
41.. Wuyts WA, Vanaudenaerde BM, Dupont LJ, N-acetylcysteine reduces chemokine release via inhibition of p38 MAPK in human airway smooth muscle cells: Eur Respir J, 2003; 22(1); 43-49
42.. Geiler J, Michaelis M, Naczk P, N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus: Biochem Pharmacol, 2010; 79(3); 413-20
43.. Zhang Q, Ju Y, Ma Y, Wang T, N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: A randomized controlled trial: Medicine (Baltimore), 2018; 97(45); e13087
44.. Mata M, Sarrion I, Armengot M, Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine: PLoS One, 2012; 7(10); e48037
45.. Poe FL, Corn J, N-Acetylcysteine: A potential therapeutic agent for SARSCoV-2: Med Hypotheses, 2020; 143; 109862
46.. Zhang Y, Ding S, Li C, Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: A meta-analysis: Exp Ther Med, 2017; 14(4); 2863-68
47.. Shin-Ichi Hagiwara YI, Kitamura Satoshi, Aerosolized administration of nacetylcysteine attenuates lung fibrosis induced by bleomycin in mice: Am J Respir Crit Care Med, 2000; 62; 225-31
48.. Huang H, Chen M, Liu F, N-acetylcysteine tiherapeutically protects against pulmonary fibrosis in a mouse model of silicosis: Biosci Rep, 2019; 39(7); BSR20190681
49.. : A study of N-acetylcysteine in patients with COVID-19 infection July 15, 2020, National Institute of Health https://clinicaltrials.gov/ct2/show/NCT04374461
50.. Wright PE, Carmichael LC, Bernard GR, Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS): Chest, 1994; 106(5); 1517-23
51.. Mancebo J, Amaro P, Lorino H, Effects of albuterol inhalation on the work of breathing during weaning from mechanical ventilation: Am Rev Respir Dis, 1991; 144(1); 95-100
52.. van der Hoeven SM, Binnekade JM, de Borgie CA, Preventive nebulization of mucolytic agents and bronchodilating drugs in invasively ventilated intensive care unit patients (NEBULAE): Study protocol for a randomized controlled trial: Trials, 2015; 16; 389
53.. Shi Q, Zhang X, Jiang F, Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: A two-center, retrospective study: Diabetes Care, 2020; 43(7); 1382-91
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250