09 June 2021: Articles 
Challenges in the Management of an Adult Snakebite Victim at a Tertiary Care Hospital: A Case Report
Unusual clinical course, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Ahmed Abdeldayem12ABCDEF*, Alhanouf Ayed Alanazi23BCDE, Jawaher N. Aljabri42BCDE, Ijaz Abid23BCDEDOI: 10.12659/AJCR.931532
Am J Case Rep 2021; 22:e931532
Abstract
BACKGROUND: The World Health Organization has set clear guidelines for the management of snakebite envenomation. However, challenges have been reported in the clinical application of guidelines, such as identification of the biting snake, hypersensitivity reactions to the antivenom, and influence of repeated antivenom administration during hospital stay. This report aims to discuss how these challenges can affect patient management and to highlight improvement opportunities.
CASE REPORT: An 18-year-old man presented to the Emergency Department without remarkable signs of envenomation following a snakebite. An initial dose of antivenom was given despite the misidentification of snake species. An allergic reaction developed and was successfully managed. Following admission, the coagulation profile and local tissue reaction worsened. Upon consulting the Drug and Poison Information Center, it was discovered that a subtherapeutic dose of antivenom was administered. The patient was rechallenged after the administration of premedication. Coagulation profile could not be maintained; therefore, 2 extra doses of antivenom were administered, resulting in sustained improvement in local tissue reaction and coagulation profile.
CONCLUSIONS: First, victims presenting without signs and symptoms of envenomation may benefit from close monitoring for late presentation of envenomation signs. Second, dosing guidelines are suggested to consider Institute of Safe Medication Practices recommendations for order sets to reduce the possibility of medication errors. Third, premedication may be an effective alternative in patients who develop allergic reaction to the locally produced equine antivenom in the setting of absent goat-derived antivenom. Lastly, antivenom administration should be titrated to patient response even if it occurs over several days.
Keywords: Compartment Syndromes, Viperidae, Medication Errors, Snake Bites, Disseminated Intravascular Coagulation, Antivenins, Emergency Service, Hospital, Horses, Length of Stay, Tertiary Care Centers
Background
Snakebite envenoming is reported in the list of neglected tropical diseases [1]. There are an estimated 2.5 million annual snake-bite envenomation cases worldwide, with up to approximately 138 000 deaths [2]. Snake fauna in Saudi Arabia includes species that can cause deadly bites, namely Arabian cobra (
Snakebite envenomation is a potentially fatal condition that results from exposure to the venom of a venomous snake either through biting or spraying into the victim’s eyes [5]. Clinical presentation of envenomation varies from local pain and swelling to severe soft-tissue reactions (eg, compartment syndrome), hematological sequelae (mainly coagulopathy), and death [6]. Coagulopathy associated with snakebite envenomation is mainly attributed to procoagulant venoms that cause activation and consumption of clotting factors, resulting in bleeding, hence the term “venom-induced consumptive coagulopathy” (VICC) [7].
Antivenom administration is a cornerstone in managing the deleterious effects of snakebite envenomation, especially local tissue reaction and coagulopathy. The presence of clinical signs and symptoms of envenomation as well as the identification of snake species play an important role in deciding whether antivenom can be used [8]. In our institution, local antivenom is produced by the National Antivenom and Vaccine Production Center and contains purified immunoglobulin fractions (F(ab’)2) prepared by hyperimmunizing healthy Arabian horses against the venoms from
Reported challenges in the management of snake poisoning include identification of the biting snake family [9] and the development of hypersensitivity reactions to the antivenom [10]. In addition, the influence of antivenom administration on the length of hospital stay is controversial [11].
This report aims to describe how the aforementioned factors affected the management of an adult snakebite envenomation victim.
Case Report
An 18-year-old man with an unremarkable past medical history presented to the Emergency Department (ED) with a snake bite injury on the back of his left big toe that had occurred 30 min before presentation. The photo of the dead snake was provided by the patient’s relative (Figure 1), who endorsed it as “Al Raqtaa”, a local synonym for
Initial laboratory investigations revealed hemoglobin of 15.7 gm/dL, white blood cell count of 5.6×109/L, platelet count of 273×109/L, international normalized ratio (INR) of 1.02, partial thromboplastin time (PTT) of 27.9 s, prothrombin time (PT) of 12.5 s, sodium of 137 mmol/L, potassium of 3.9 mmol/L, blood urea nitrogen of 3.7 mmol/L, and serum creatinine of 73 mcmol/L. The chest X-ray was normal; however, venous Doppler ultrasound of the lower limb showed subcutaneous edema and bilateral lymph node enlargement in the thigh area, without evidence of venous thrombosis.
Although the initially endorsed snake name was not included in the spectrum of activity of the local ASV, the physician decided to administer the antivenom after premedicating the patient. Even though neither the product information nor the practice guidelines [8] recommend antihistamines or corticosteroids as routine premedication, the patient was prescribed 120 mg methylprednisolone and 50 mg diphenhydramine intravenously (i.v.) as premedication. While preparing the diluted ASV solution in the hospital pharmacy, an initial dose of 10 mL undiluted ASV was ordered and administered over 3 min. When the diluted solution of ASV (40 mL of ASV in 250 mL of normal saline) arrived, a verbal order was given to administer only 30 mL based on ASV content, which is equivalent to 187.5 mL of the diluted solution. However, the order was mis-interpreted and only 30 mL of the total volume of diluted solution was administered. The patient developed a skin reaction in the form of swelling and rash over the face, which was managed by administering epinephrine 0.3 mg intramuscular-ly. Afterward, the patient was admitted to the general medical ward for observation.
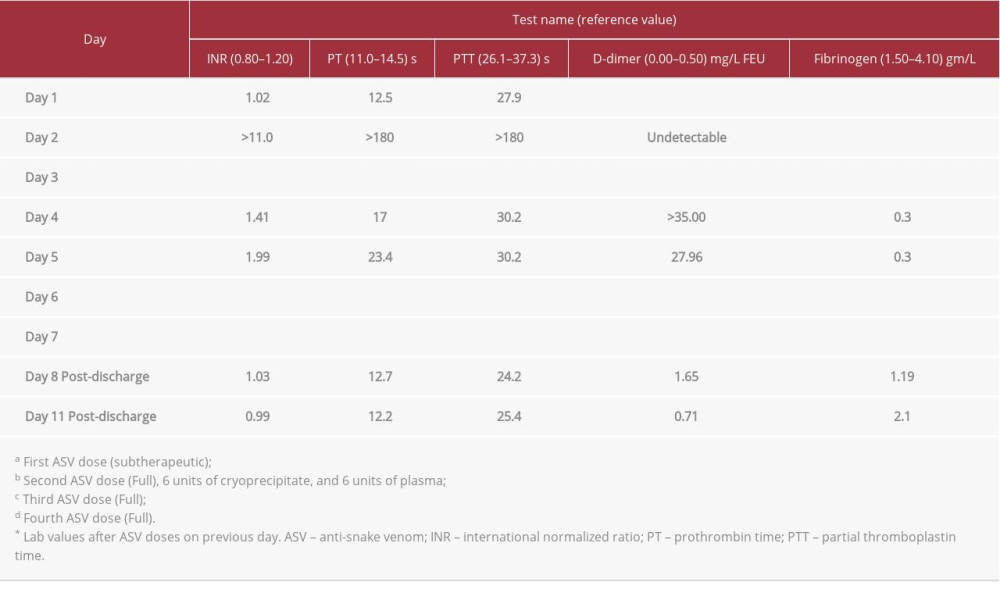
Eight hours later, the patient was hemodynamically stable except for minimal bleeding from the venipuncture site and gums, with no bleeding from any other site. INR, PT, and PTT values were unmeasurably high. Fibrinogen was <0.30 gm/L, and the D-dimer level was not detectable (Table 1). These findings were consistent with the hematological sequelae of snakebite envenomation. A peripheral blood smear showed no abnormal morphology. Swelling and inflammation were observed in the bitten limb.
Upon consultation with the Drug and Poison Information Center on day 2, the error in the administered dose was discovered. Therefore, ASV redosing was recommended by the Drug and Poison Information Center based on the diagnosis of snakebite envenomation with local tissue reaction and VICC. After discussing the case with the patient and the patient’s relative and explaining the benefits of ASV administration versus the possible allergic reaction, the patient agreed to be rechallenged with ASV. He received 0.3 mg subcutaneous epinephrine and 100 mg i.v. hydrocortisone followed by a more diluted ASV dose at a slower rate of infusion (40 mL of ASV in 300 mL of normal saline over 3 h). Moreover, piperacillin/tazobactam and vancomycin were prescribed empirically for what was suspected to be bacterial cellulitis rather than a local tissue reaction from envenomation. In addition, 6 units of fresh frozen plasma were given on day 2, and 6 units of cryoprecipitate were given on days 2 and 3.
The patient’s coagulation profile improved initially, but it worsened again over days 3 and 4. Therefore, premedication followed by 2 doses of ASV were administered on days 5 and 6, which resulted in significant improvement in the coagulation profile (Table 1) and local tissue reaction (Figures 3, 4). The patient was discharged on day 7, and follow-up laboratory investigations on days 8 and 11 showed a maintained improvement in the coagulation profile.
Following patient discharge, a local snake expert identified the snake by the chess-like pattern on the body to have been an
Discussion
The WHO guidelines for the management of snakebite envenomation emphasize essential clinical roles: patient assessment and stabilization, snake identification, laboratory tests, anti-venom administration, and management of possible allergic reaction to ASV [8]. However, implementation of these guidelines in real-world situations can be challenging.
Since ASVs are specific in their action [13], snake identification plays a key role in determining the need for antivenom administration. In our case, the snake species was misidentified by the patient’s relative; however, the first dose of ASV was administered in the ED as a standard precautionary measure. When laboratory results showed significant abnormal coagulation indices (PT, PTT, INR), the decision to readminister ASV was challenging, especially in the context of a questionable snake species and risk of anaphylaxis based on the patient’s response to the initial dose. Nevertheless, the reported possible cross-activity of ASVs in the WHO recommendation [8] and the impending risk of bleeding favored the decision to re-administer the ASV on day 2. After the ASV had been administered, the coagulation profile showed initial improvement.
The patient did not present with remarkable signs or symptoms of envenomation. Although the patient had no other contributing medical conditions or medications, the coagulation profile and local tissue reaction worsened after admission, which was attributed to envenoming. If the patient had been discharged based on the initial laboratory results and near-normal presentation, his outcome would have been different. Therefore, it may be beneficial to put patients who present with no clinical signs of envenomation under close medical observation.
The administration of a subtherapeutic dose of ASV in the ED was investigated to identify the root cause. The Institute of Safe Medication Practices (ISMP) recommends excluding order sets that express the dose of drugs in volume (mL) or number of vials [14]. However, the dose of ASV is commonly expressed in the literature [15,16] as well as in WHO guidelines [8] in these units, which contradicts the former safety recommendation. This variability in dose designation may present an inherent cause of process failure [17] that can take place in healthcare systems with multicultural practitioners who have different practice backgrounds. Accordingly, it is recommended to use the total content of units/mg of antivenom to express the dose rather than the volume.
In the present case, we involved the patient in decision making after proper counseling [18]. After the dose on day 2, the primary team decided to adopt a watch and wait approach to avoid the risk of anaphylaxis. Upon further deterioration of the coagulation profile, and after clarifying the risk of impending bleeding versus the risk of an allergic reaction, the patient eventually accepted the risk of readministering ASV on day 5. The administration of 2 consecutive doses of ASV was associated with achieving and maintaining the improvement in the coagulation profile.
Coagulopathy and local soft-tissue reaction were the prominent manifestations of envenomation in our patient. VICC is a prevalent cause of hematological manifestations of snakebite envenomation that results from the procoagulant properties of venom, which manifested in our patient as the depletion of fibrinogen and other coagulation factors, ultimately resulting in prolonged PT, PTT, and INR [7]. The ASV dose on day 2 was effective in neutralizing the venom effect as evidenced by the improvement in the coagulation profile; however, this effect was not maintained. WHO guidelines suggest that anti-venom can be administered up to 10 days after a bite because of the release of venom from the bite site into the circulation. As coagulation indices worsened in our patient over days 3 to 5, the ASV dose was repeated. This observation matched the basic pharmacokinetics of venom [19], which supports the recommendation to continue monitoring the coagulation profile after initial improvement with ASV administration [20].
In addition to the improvement in the coagulation profile in our patient, the ASV might have influenced the resolution of the local soft-tissue reaction. Administration of antibiotics did not result in improvement of the local tissue reaction, which favored the diagnosis of an impending compartment syndrome, a documented complication of snakebite envenomation [21,22], rather than cellulitis. Upon ASV administration on day 5, marked improvement in local swelling was observed, as illustrated in Figures 3 and 4. This conforms with the available evidence about the efficacy of ASV in managing local soft-tissue reaction and halting the progression to compartment syndrome.
Although the product information recommends switching to goat-derived ASV in cases of allergic reaction, administration of diluted ASV solution over a longer period, subcutaneous epinephrine, and i.v. hydrocortisone were successful in preventing the recurrence of an allergic reaction. These measures may provide an alternative when goat-derived ASV is not available. However, it is worth mentioning that, other than subcutaneous epinephrine, the efficacy of these measures in preventing allergic reaction is controversial [23].
The aforementioned factors likely prolong the length of hospital stay (LOS), which may explain the gap in the available evidence. Keung et al found that repeated doses of ASV have been associated with longer LOS [11]. This is likely due to the severity of the snakebite envenomation rather than the efficacy of repeated ASV dosing. In their study, almost 66% of the patients who received multiple doses had grade IV or V injury, compared with only 25% of the patients who received a single dose; therefore, the LOS might have been affected by the injury level rather than by the number of ASV doses. In our case, the ASV administration was held from day 2 to day 5 to avoid the risk of a possible anaphylactic reaction, which resulted in the extension of LOS to 7 days. Earlier administration of subsequent ASV doses might have resulted in the earlier improvement and discharge of the patient.
Conclusions
This case highlights the challenging nature of snakebite envenomation management. First, victims who present without signs and symptoms of envenomation may benefit from close monitoring for late presentation of envenomation signs. Second, dosing guidelines are suggested to consider ISMP recommendations for order sets to reduce the possibility of medication errors. Third, premedication may be an effective alternative in patients who develop an allergic reaction to the locally produced equine antivenom in the absence of a goat-derived antivenom alternative. Moreover, involvement of a specialized poison information center can result in better management of snakebite envenomation. Lastly, ASV administration should be titrated to patient response even if it occurs over several days.
References:
1.. Chippaux J-P, Snakebite envenomation turns again into a neglected tropical disease!: J Venom Anim Toxins Incl Trop Dis, 2017; 23; 38
2.. Williams DJ, Faiz MA, Abela-Ridder B, Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming: PLoS Negl Trop Dis, 2019; 13(2); e0007059
3.. Ismail M, Memish ZA, Venomous snakes of Saudi Arabia and the Middle East: A keynote for travellers: Int J Antimicrob Agents, 2003; 21(2); 164-69
4.. Al-Sadoon MK, Snake bite envenomation in Riyadh province of Saudi Arabia over the period (2005–2010): Saudi J Biol Sci, 2015; 22(2); 198-203
5.. , Snakebite envenoming. [cited 17 April 2021] https://www.who.int/snakebites/disease/en/
6.. Al-Durihim H, Al-Hussaini M, Bin Salih S, Snake bite envenomation: Experience at King Abdulaziz Medical City, Riyadh: East Mediterr Health J, 2010; 16(4); 438-41
7.. Isbister GK, Scorgie FE, O’Leary MA, Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10): J Thromb Haemost, 2010; 8(11); 2504-13
8.. Tempowski JH: Guidelines for the prevention and clinical management of snakebite in Africa, 2010, Regional Office for Africa, WHO
9.. Williams HF, Vaiyapuri R, Gajjeraman P, Challenges in diagnosing and treating snakebites in a rural population of Tamil Nadu, India: The views of clinicians: Toxicon, 2017; 130; 44-46
10.. de Silva HA, Ryan NM, de Silva HJ, Adverse reactions to snake antivenom, and their prevention and treatment: Br J Clin Pharmacol, 2016; 81(3); 446-52
11.. Park KH, Shin H, Kang H, Effectiveness of repeated antivenom therapy for snakebite-related systemic complications: J Int Med Res, 2019; 47(10); 4808-14
12.. Obeidat MB, Al-Swailmeen AM, Al-Sarayreh MM, Rahahleh KM: Am J Case Rep, 2020; 21; e922000
13.. Pucca MB, Cerni FA, Janke R, History of envenoming therapy and current perspectives: Front Immunol, 2019; 10; 1598
14.. , IG for SOS. Guidelines for Standard Order Sets, 2010 https://www.ismp.org/guidelines/standard-order-sets
15.. Gajbhiye R, Khan S, Kokate P, Incidence & management practices of snakebite: A retrospective study at Sub-District Hospital, Dahanu, Maharashtra, India: Indian J Med Res, 2019; 150(4); 412-16
16.. Alirol E, Sharma SK, Ghimire A, Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: Evidence from a randomised controlled trial in Nepal: PLoS Negl Trop Dis, 2017; 11(5); e0005612
17.. Bates DW, Vanderveen T, Seger D, Variability in intravenous medication practices: Implications for medication safety: Jt Comm J Qual Patient Saf, 2005; 31(4); 203-10
18.. Greene S, Do we know the correct dose of tiger snake antivenom?: Emerg Med Australas, 2019; 31(4); 504-5
19.. Sanhajariya S, Duffull SB, Isbister GK, Pharmacokinetics of snake venom: Toxins (Basel), 2018; 10(2); 73
20.. Jeon YJ, Kim JW, Park S, Shin DW, Risk factor, monitoring, and treatment for snakebite induced coagulopathy: A multicenter retrospective study: Acute Crit Care, 2019; 34(4); 269-75
21.. Bucaretchi F, De Capitani EM, Hyslop S: Clin Toxicol (Phila), 2014; 52(6); 639-41
22.. Hsu C-P, Chuang J-F, Hsu Y-P, Predictors of the development of post-snakebite compartment syndrome: Scand J Trauma Resusc Emerg Med, 2015; 23; 97
23.. : Guidelines for the management of snakebites, 2016, egional Office for South-East Asia, WHO
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250