01 November 2021: Articles 
Early Gestational Age Placenta Accreta Case Report: Can We Avoid Missed Diagnosis?
Mistake in diagnosis
Sigit Purbadi1ABD*, Hartono Tjahjadi2BD, Gatot PurwotoDOI: 10.12659/AJCR.934168
Am J Case Rep 2021; 22:e934168
Abstract
BACKGROUND: Placenta accreta is an abnormal invasive placenta that can be life-threatening because of the risk of hemorrhage. Its incidence has increased due to high cesarean delivery rates. Early gestational age placenta accreta is difficult to diagnose and misdiagnosis can lead to inappropriate treatment.
CASE REPORT: Patient 1, a 34-year-old woman (para 2 abortus 1) with 2 previous cesarean deliveries, was referred to our department for vaginal bleeding and abdominal pain. She received 2 curettages for blighted ovum; then, ultrasound examination found uterus perforation and fluid in the Douglas cavity. Exploratory laparotomy confirmed uterine perforation, and a hysterectomy was performed. Histopathological examination revealed placenta accreta. Patient 2, a 35-year-old woman (para 3) with 3 previous cesarean deliveries, was treated at a previous hospital for vaginal bleeding and stomach enlargement. She received serial chemotherapy for gestational trophoblastic neoplasia. Ultrasound examination showed a nonhomogeneous opacity in the lower uterine corpus with color score 4. Total abdominal hysterectomy was performed, and histopathological examination revealed placenta accreta. Patient 3, a 32-year-old woman (para 2) with 2 previous cesarean deliveries, had irregular vaginal bleeding suspected as gestational trophoblastic neoplasia due to ultrasound examination and positive beta-human chorionic gonadotropin. Ultrasound and MRI examination showed enlargement with nonhomogeneous opacity, color score 4, and bridging vessels. Due to our previous experience, we suspected it was a placenta accreta and performed a hysterectomy. The histopathology result indicated placenta accreta.
CONCLUSIONS: The key point in diagnosing placenta accreta properly is to evaluate the morphometric changes based on the structure using imaging like ultrasound. Collection and analysis of these data enables precise diagnosis in early gestational age placenta accreta.
Keywords: Cesarean Section, Diagnostic Techniques, Obstetrical and Gynecological, Placenta Accreta, Female, Gestational Age, Humans, Hysterectomy, Missed Diagnosis, Placenta, Pregnancy
Background
Placenta accreta is an abnormally invasive placenta that can be life-threatening due to hemorrhage [1]. It occurs when there is steadfast adherence to the uterine wall [2]. As a consequence of partial or total absence of the decidua basalis and imperfect development of the fibrinoid or Nitabuch layer, placental villi become attached to the myometrium in placenta accreta. As a result, at least part of the placenta cannot separate after delivery and may lead to severe obstetric hemorrhage [3].
Placenta accreta prevalence is now estimated to be 1 in 2500 pregnancies [4], but the incidence has increased every year following increases in cesarean delivery rates [5]. Because of the possibility of complications, we need to identify placenta accreta during the first trimester or as soon as possible. Early diagnosis is critical for directing clinical management and for preventing associated immediate complications.
Ultrasound is the primary diagnostic modality during the ante-partum period and the modality of choice to evaluate obstetrical hemorrhage [6]. Morphometric data from the uterine wall, especially in the niche area and trophoblast structure, provide the necessary clue to make a proper diagnosis in the early gestational stage. Combined grayscale and color Doppler ultrasound allows the assessment of the uterine cavity and its blood flow [7]. We also can use beta- gonadotropin (β-hCG) levels if our examination leads to suspicion of gestational trophoblastic disease [8]. Early gestational age is the stage at which placenta accreta is usually misdiagnosed. Leading causes of variability that may result in misdiagnosis is an important issue that should be discussed.
Herein, we present 3 cases of placenta accreta in early gestational age: 1 that was misdiagnosed as missed abortion, 1 that was misdiagnosed as gestational trophoblastic neoplasia (GTN), and 1 that was, due to our previous experience, correctly diagnosed as placenta accreta.
Case Reports
A 34-year-old woman, para 2 abortus 1, with histories of 2 previous cesarean deliveries and 2 previous curettages, was referred for vaginal bleeding. She had no idea whether she was pregnant or not. She complained of having severe abdominal pain 2 weeks before admission to a hospital, where she received curettage in March 2021 due to blighted ovum. One day after curettage, she was discharged, but 1 week later she reported spotting. Despite treatment, the spotting did not stop, and she was referred to a second hospital. The ultrasonography examination found an intrauterine mass and then did the second curettage in April 2021. A week after this second curettage, the patient reported fever and spotting, which worsened, so she was taken to the emergency room (ER). She was treated by internal medicine, given antibiotics and an anal-gesic, but there was no improvement after 6 days of therapy. The patient was then referred to us due to suspected pelvic infection and adhesion to the intestine.
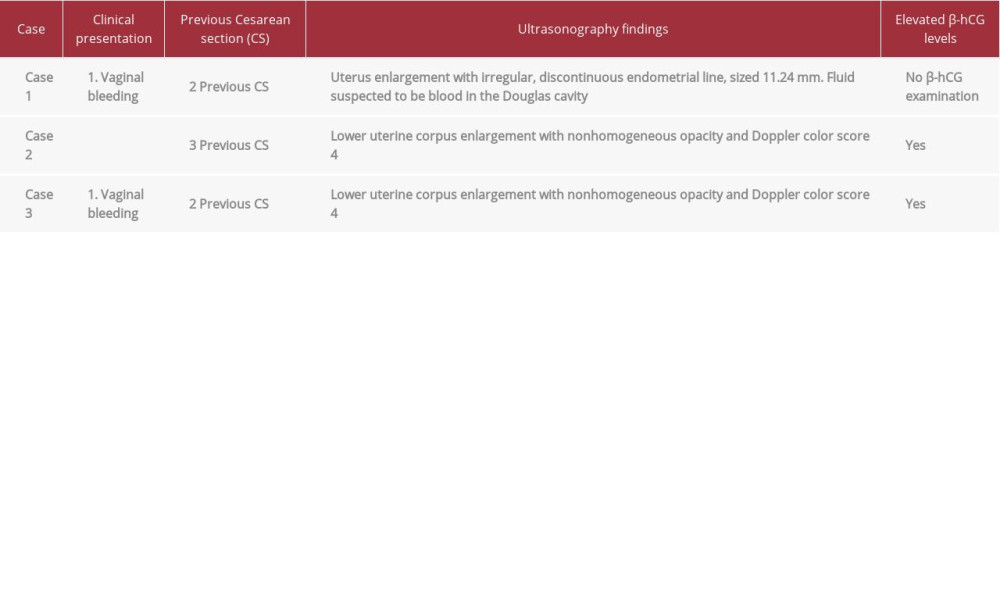
In our ER, the patient arrived with hypovolemic shock grade I and abdominal pain. After she was stabilized, we performed an ultrasound examination and found uterus enlargement with irregular and discontinuous endometrial lining, which was suspected to be a sign of uterus perforation, sized 11.24 mm at the anterior corpus uteri near the isthmus, and we also saw fluid, suspected to be blood, filling part of the lesion and extending to the Douglas cavity. From the examination, we planned an exploratory laparotomy after the patient’s vital signs became stabilized. In the exploratory laparotomy, we confirmed our findings that there was perforation in the uterine lining, so we decided to do a hysterectomy. We did a histopathology examination from the hysterectomy sample, and the results revealed a placenta accreta (Figure 1). The postoperative condition of the patient was stable. She received antibiotic treatment, and was discharged on postoperative day 4.
In the second case, a 35-year-old, para 3 woman was admitted to a primary health care facility with sudden vaginal bleeding. Her obstetric history included 3 previous cesarean section procedures and no previous miscarriages. The obstetrician/ gynecologist believed that she had experienced a spontaneous abortion. Ultrasonography examination indicated a mass in the myometrium, so the doctor did not consider curettage for this case. The patient received medication and planned to continue the therapy in the outpatient setting as followup.
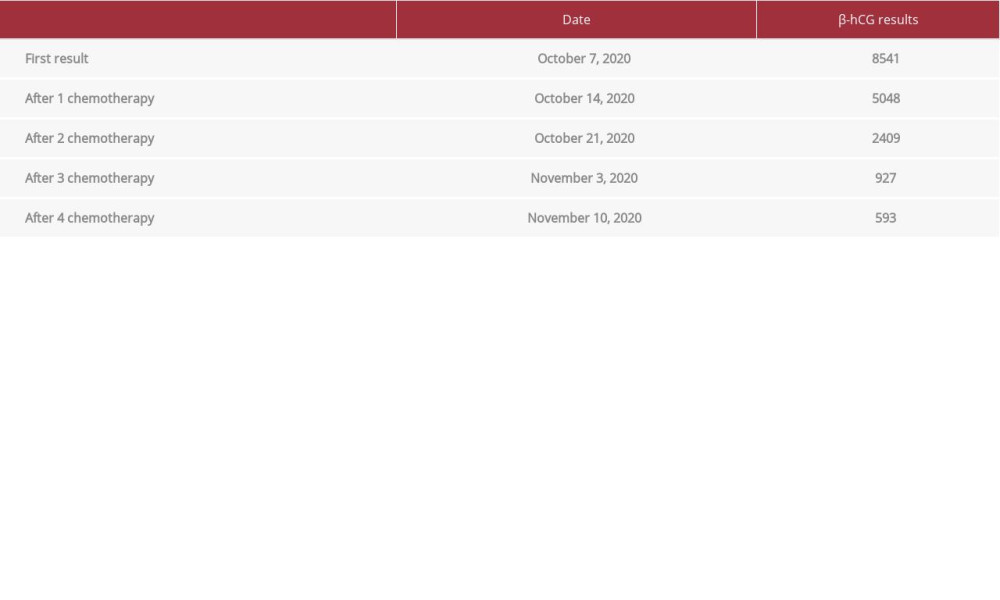
During the 1-month followup, the patient said that she felt her stomach was getting bigger, but no vaginal bleeding discharge and no abdominal pain were reported. She then consulted with an obstetrician/gynecologist and was referred to a gyneco-logic oncologist in a second hospital. The doctors did a series of examinations, from physical to imagining. The ultrasound examination showed a mass in the uterine corpus. The doctors checked the β-hCG levels to support the diagnosis, and the first β-hCG examination showed a massive concentration. Because of all of these findings, the doctor gave a diagnosis of GTN. The doctor then suggested the patient undergo 4 cycles of chemotherapy and received serial β-hCG examination after the chemotherapy (Table 1).
The patient agreed, and she received 4 cycles of chemotherapy with methotrexate. The followup examination showed reduced β-hCG levels, but the tumor did not resolve, so the patient was referred to our hospital. In our hospital, we did a re-examination to confirm the diagnosis. We observed enlargement of the lower uterine corpus from our ultrasound examination, with a nonhomogeneous opacity in the lower uterine corpus. The Doppler showed a color score of 4. We did not perform advanced imaging like computed tomography (CT scan) or magnetic resonance imaging (MRI) because we thought there was enough information to diagnose this patient with placenta accreta and choose the correct therapy.
From our examination, we suggested the patient undergo operative therapy for further management. The patient agreed and performed a total abdominal hysterectomy, and the mass was sent to the pathology-anatomy department for a histo-pathology examination. The histopathology result confirmed that it was a placenta accreta (Figure 2). The postoperative recovery was uncomplicated, and the patient was discharged on postoperative day 2.
In the third case, a 32-year-old woman, para 2, with a history of 2 cesarean deliveries, was referred for vaginal bleeding. She had no idea whether she was pregnant or not. She said that she had been experiencing irregular vaginal bleeding for almost 4 months, since December 2020. On the basis of the ultrasound examination and positive β-hCG result, the obstetrician/gynecologist diagnosed the condition as GTN.
The patient was then referred to us. From the physical examination, we saw a normal cervix with light or non-active bleeding and uterus enlargement. The ultrasonography examination showed an enlargement of the lower part of the uterine corpus with nonhomogeneous opacity, and the Doppler examination showed neovascularization with a color score of 4 (Figure 3). We also did advanced imagining, and the MRI results supported our ultrasound-based examination with a nonhomogeneous mass and enlargement of the lower uterine corpus with a bridging vessel that was suspected to be invasive to the bladder.
The examination results gave a first impression that this was still a case of malignant trophoblastic neoplasia. Then, we assessed β-hCG levels. The β-hCG level was 2504, so we sought to differentiate the diagnosis, between GTN (choriocarcinoma or placenta trophoblastic tumor) and placenta accreta. From our previous experience, we had seen patients like this, who had suffered from placenta accreta, so we moved placenta accreta to be the most probable diagnosis for this patient.
Management options included chemotherapy and an operative procedure, but we suggested that the patient undergo a hysterectomy procedure due to inadequate response to chemotherapy. The patient and her husband did not desire future fertility and preferred hysterectomy as we suggested.
The patient underwent a total abdominal hysterectomy. The macroscopic sample was then referred to the pathology-anatomy department for a histopathology examination. The pathology examination demonstrated placenta accreta (Figure 4). The postoperative course was uncomplicated, and the patient was discharged on postoperative day 2.
Discussion
Placenta accreta is an invasive placental condition giving rise to a life-threatening obstetrical emergency when it causes hemorrhage [1]. It occurs when there is abnormally firm adherence to the uterine wall. In placenta accreta, the placenta has infiltrated into the superficial area of the basalis layer [3,9]. As a consequence of partial or total absence of the decidua basalis and imperfect development of the fibrinoid or Nitabuch layer, placental villi become attached to the myometrium. As a result, at least part of the placenta cannot separate after delivery and this may lead to severe obstetric hemorrhage [2,3]. That is why proper diagnosis is needed to choose the right treatment, and for this, it is necessary to precisely understand the pathological structure, to make an appropriate diagnosis.
Placenta accreta prevalence is now estimated to be 1 in 2500 pregnancies [4]. The incidence has risen in parallel with that of cesarean deliveries, and it remains a major cause of maternal mortality and morbidity and the principal indication for postpartum hysterectomy [5,10,11].
Antenatal diagnosis of invasive placentation is critical and has been shown to decrease maternal morbidity [12]. Ultrasonography is the primary diagnostic modality during the antepartum period and the modality of choice to evaluate for obstetrical hemorrhage, with a sensitivity of 91% and specificity of 97%. Some recent studies have described sono-graphic characteristics of placenta accreta in the first trimester as a low-lying gestational sac and diffuse dilatation of intra-placental vessels (lacunae) traversing the lower uterine segment at the site of the cesarean section scar [13,14]. The Royal College of Obstetricians and Gynecologists (RCOG) guidelines for placenta accreta describe the signs as the following findings on 2D greyscale Doppler: loss of the ‘clear zone’, abnormal placental lacunae, bladder wall interruption, myometrial thinning, placental bulge, and focal exophytic mass. On the other hand, on 2D color Doppler, we can find uterovesical hypervascularity, subplacental hypervascularity, bridging vessels, and placental lacunae feeder vessels [15]. Combined grayscale and color Doppler ultrasound allows assessment of the uterine cavity and its blood flow [16,17]. MRI can be used to support the diagnosis or as a diagnostic adjunct in complicated cases. The RCOG recommendations also state that the diagnostic value of MRI and ultrasound imaging in placenta accreta spectrum is similar when performed by experts. MRI can be used to complement ultrasound imaging to assess the depth of invasion and lateral extension of myometrial invasion [15,18].
Gestational trophoblastic disease is the term used to encompass a group of tumors typified by abnormal trophoblastic proliferation that produce β-hCG. GTN, the malignant form of gestational trophoblastic disease, includes invasive mole, choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor. Each of the GTN malignancy types is histologically distinct and varies in its propensity to invade and metastasize [3,19]. Instead of histology of a specimen, measurement of serum β-hCG levels combined with clinical findings is used to diagnose and treat this malignancy [3,8]. In 2 of our cases, the preliminary diagnosis was GTN because of the similarity of the patients’ sonographic and laboratory findings (β-hCG at massive levels). Early-stage GTN is typically cured with a single chemotherapy agent [20]. In our second patient, the doctor in the previous hospital used methotrexate single chemotherapy as treatment.
GTN infiltrates into the myometrium in a nonspecific area, while placenta accreta infiltrates into the myometrium in a specific location, especially in the anterior isthmus part of the uterine lining that we call the niche. Most cases of placenta accreta are initiated from a pregnancy located in the isthmus or uterine niche, which occurs due to scars [3,9,21]. In this case, we had pathological findings of a mass in the isthmus that had spread into the lower uterine corpus, and from the appearance of the Doppler/ultrasonography, we also saw a mass in the lower uterine corpus, extending from the isthmus with non-homogeneous opacity and high vascularity. From these findings, we concluded that although placenta accreta can mimic a GTN, with the same laboratory and clinical findings, this case was more like a placenta accreta than a GTN because GTN does not have a specific location of invasion.
This report illustrates that it is crucial to consider the diagnosis of retained invasive placenta like placenta accreta in patients presenting with vaginal hemorrhage who have a history of previous cesarean delivery. The use of histopathological examination is essential to help us avoid misdiagnosis.
In the first case presented here, the niche and the cervix were never evaluated, which is important because this is where the trophoblast is implanted. On the other hand, from the second case, we know that the anterior wall of the uterus was also not evaluated. This patient was diagnosed with GTN, but the invasion was only in the anterior wall of the uterus. These findings, retrospectively, indicated that they were more likely to be placenta accreta cases than GTN cases. Our conclusion for the second case was supported by the histopathology results, which told the same story. In the last case, with our experiences of misdiagnosis from the first and second cases, because of the uterine anterior wall trophoblast invasion problem, we knew from the history, physical examination, ultrasounds, and MRI findings that it was a case of placenta accreta, and this was confirmed by the histopathology results (Table 2). The consequences of missed diagnosis in the first and second cases led to unnecessary treatment and also complications like uterine rupture due to curettages and chemo-therapy for the second patient. The clue to consider to make a proper diagnosis is niche and low-lying placenta, which can be detected based on the histological structure viewed via imaging in ultrasounds or MRI. We should carefully inspect the anterior uterine wall, down to the cervix, and also the tropho-blast structure, including the invasiveness, every time we do an ultrasound examination. Careful evaluation is needed for better diagnosis, as cesarean scar pregnancy with wide anterior uterine wall trophoblast invasion cannot be resected. In this situation, hysterectomy is the correct treatment.
Conclusions
Early gestational age placenta accreta can be appropriately diagnosed. For every pregnancy after a previous cesarean section, the doctor must evaluate the anterior isthmus to look for a niche. Careful examination is needed to diagnose placenta accreta and to determine proper management. Both imaging and histopathological examination play important roles in making the diagnosis. The key point is to evaluate whether the trophoblast is invading the anterior wall in the isthmus area or not. This method can be used to evaluate whether the pregnancy was normal or potentially became placenta accreta or cesarean scar pregnancy. It is vital to know how to differentiate placenta accreta from GTN, although both can show increased β-hCG levels. From imaging examination, we can determine that placenta accreta is primarily located in the isthmus or uterine niche that occurs due to scars, while GTN has no specific area. On the imaging examination, we should inspect the niche; specifically looking for growth to a larger size. The morphometric changes based on the structure and its invasion can be evaluated by ultrasound or MRI. By collecting and analyzing these data, we can make a precise diagnosis in early gestational age placenta accreta.
Figures
References:
1.. Angstmann T, Gard G, Harrington T, Surgical management of placenta accreta: A cohort series and suggested approach: Am J Obstet Gynecol, 2010; 202(1); 38.e1-e9
2.. Silver RM, Landon MB, Rouse DJ, Maternal morbidity associated with multiple repeat Cesarean deliveries: Obstet Gynecol, 2006; 107(6); 1226-32
3.. Cunningham F, Kenneth J, Bloom S, Al E: Williams obstetric, 2014, New York, McGraw-Hill Companies
4.. Miller DA, Chollet JA, Goodwin TM, Clinical risk factors for placenta previa – placenta accreta: Am J Obstet Gynecol, 1997; 177(1); 210-14
5.. Shellhaas CS, Gilbert S, Landon MB, The frequency and complication rates of hysterectomy accompanying Cesarean delivery: Obstet Gynecol, 2009; 114(2); 224-29
6.. Abramowicz JS, Sheiner E, In utero imaging of the placenta: Importance for diseases of pregnancy: Placenta, 2007; 28; S14-22
7.. Sellmyer MA, Desser TS, Maturen KE, Physiologic, histologic, and imaging features of retained products of conception: Radiographics, 2013; 33(3); 781-96
8.. Cole LA, hCG, its free subunits and its metabolites. Roles in pregnancy and trophoblastic disease: J Reprod Med, 1998; 43(1); 3-10
9.. Jauniaux E, Collins S, Burton GJ, Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging: Am J Obstet Gynecol, 2018; 218(1); 75-87
10.. Briery CM, Rose CH, Hudson WT, Planned vs emergent cesarean hysterectomy: Am J Obstet Gynecol, 2007; 197(2); 154.e1-e5
11.. Eller AG, Bennett MA, Sharshiner M, Maternal morbidity in cases of placenta accreta managed by a Multidisciplinary Care Team compared with standard obstetric care: Obstet Gynecol, 2011; 117(2); 331-37
12.. Warshak CR, Ramos GA, Eskander R, Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta: Obstet Gynecol, 2010; 115(1); 65-69
13.. Ben Nagi J, Ofili-Yebovi D, Marsh M, Jurkovic D, First-trimester Cesarean scar pregnancy evolving into placenta previa/accreta at term: J Ultrasound Med, 2005; 24(11); 1569-73
14.. Yang JI, Kim HY, Kim HS, Ryu HS, Diagnosis in the first trimester of placenta accreta with previous Cesarean section: Ultrasound Obstet Gynecol, 2009; 34(1); 116-18
15.. Jauniaux E, Alfirevic Z, Bhide A, Placenta praevia and placenta accreta: Diagnosis and management: BJOG, 2019; 126(1); e1-48
16.. Calì G, Giambanco L, Puccio G, Forlani F, Morbidly adherent placenta: Evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta: Ultrasound Obstet Gynecol, 2013; 41(4); 406-12
17.. D’Antonio F, Iacovella C, Bhide A, Prenatal identification of invasive placentation using ultrasound: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2013; 42(5); 509-17
18.. Elsayes KM, Trout AT, Friedkin AM, Imaging of the placenta: A multi-modality pictorial review: Radiographics, 2009; 29(5); 1371-91
19.. Lurain JR, Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole: Am J Obstet Gynecol, 2010; 203(6); 531-39
20.. May T, Goldstein DP, Berkowitz RS, Current chemotherapeutic management of patients with gestational trophoblastic neoplasia: Chemother Res Pract, 2011; 2011; 806256
21.. Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, Outcome of Cesarean scar pregnancy managed expectantly: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2018; 51(2); 169-75
Figures
In Press
22 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943346
24 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943560
26 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943893
27 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942126
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250