22 February 2022: Articles 
Implication of Genetic Testing and Pregnancy Outcome in a Woman with Unbalanced Translocation t(1;6)
Diagnostic / therapeutic accidents, Rare disease
Marija Jurčenko1BCEF*, Madara Auzenbaha21AE, Ieva Mičule1DE, Ieva Grīnfelde21AE, Aigars Dzalbs1D, Ieva Mālniece1ADOI: 10.12659/AJCR.935370
Am J Case Rep 2022; 23:e935370
Abstract
BACKGROUND: Parental chromosomal structural abnormalities can lead to diverse chromosomal imbalances at meiotic segregation during gametogenesis and subsequent early pregnancy loss or birth of a child with a chromosomal abnormality. The incidence of unbalanced translocations is 1 per 1000 newborns versus 3 per 1000 newborns for balanced rearrangements. Here, we present the case of a mother with an unbalanced chromosomal translocation and her offspring.
CASE REPORT: Our patient had a 1p36.31 duplication of 0.22 Mb and 6qter deletion of 1.2 Mb. She had 5 pregnancies with different outcomes. Her first child died 24 h after birth due to a congenital heart defect. Her second pregnancy resulted in the birth of a girl who was postnatally diagnosed with 1p36 deletion syndrome. The third and fourth pregnancies ended spontaneously in the first trimester. For her last pregnancy, the patient underwent a diagnostic amniocentesis at the 16th week of gestation. A large 5.4-Mb pathogenic duplication of 1p36.33 was detected in the fetus and the woman decided to terminate the pregnancy.
CONCLUSIONS: In this case report, we detail the different pregnancy outcomes induced by the mother’s unbalanced chromosomal translocation and review the prenatal diagnostic genetic testing. Our report clearly demonstrates the complementary nature of chromosomal microarrays and conventional karyotyping.
Keywords: Microarray Analysis, Abnormal Karyotype, Translocation, Genetic, Amniocentesis, Child, Chromosome Disorders, Female, genetic testing, Humans, Infant, Newborn, Pregnancy, Pregnancy Outcome
Background
The present report describes the clinical features of a woman with unbalanced translocation t(1;6) and her observed pregnancy outcomes. We detail the several chromosomal aberrations involving chromosomes 1 and 6 detected in her family. Consulting the current literature, we found that the clinical significance of 6q terminal deletion can vary and that deletion may occur either de novo or due to inherited non-reciprocal translocations, involving already described rearrangements between chromo-some 6 and 1, 3, 10, 17, 18, 21, 22 autosomes. Although 6qter deletion is seldomly described in the literature, it has been shown to be related to structural brain abnormalities and intellectual disability in children. Clinical signs of 6q terminal deletion syndrome – epileptic seizures, facial dysmorphism, joint hypermobility, and hypotonia – have recently been described [1].
1p36 deletion syndrome is one of the most common terminal deletions detected, occurring in 1 in 5000 births [2]. Due to terminal deletions and haploinsufficiency of a number of genes, it has an observable phenotypic appearance. It has been reported that up to 16% of 1p36 monosomies are due to unbalanced translocations [3]. It is known that 80% of the first chromosome terminal deletions have a maternal origin [4,5]. The length of the deletion is extremely variable and although chromosomal breaks in the region 1p36.13–1p36.33 are frequent, there is no single common breakpoint. It is difficult to identify a phenotype-genotype correlation among 1p36 deletion syndrome individuals because of the variable size of the deleted segment and even variable clinical manifestations of similarly sized deletions [6]. Nevertheless, the main clinical features are developmental delay, intellectual disability, epileptic seizures, brain anomalies, vision and hearing problems, short stature, facial dysmorphism (characteristically straight eyebrows with slightly deep-set eyes), orofacial clefting, congenital heart defects, dilated cardiomyopathy, and renal anomalies [7]. Although there are no specific prenatal signs associated with 1p36 deletion syndrome, it is recommended that observation of ventriculomegaly, congenital heart defect, or facial dysmorphism should signal consideration of 1p36 deletion syndrome [8].
1p36 microduplication is a rare multisystemic pathology characterized by intellectual disability, microcephaly, epileptic seizures, and polymicrogyria [9]. Further clinical signs include developmental delay, mild facial dysmorphism, and neurological, cardiac, and skeletal anomalies [10]. The DECIPHER database contains a few cases of 1p36 microduplication larger than 5 Mb, which add velopharyngeal insufficiency, syndactyly, and autism to the list of clinical features.
Case Reports
We report the case of a female patient who was referred to the Prenatal Diagnostic Center during her second pregnancy due to a previous adverse pregnancy outcome (Figure 1).
The index case. It is known that she had delayed motor development at an early age. She received a special education and became a professional dyer. She is currently 35 years old and reports having episodes of memory decline. The patient had osteoarthritis in both knees, both of which have been operated on. Her obstetric medical history is detailed in Table 1. During her second pregnancy, karyotype analysis (400-band resolution) and fluorescence in situ hybridization (FISH) 22q11.2 did not reveal any pathology. Following detection of an unbalanced translocation in her child, a subtelomeric FISH analysis was conducted. The results revealed an unbalanced trans-location between chromosomes 1 and 6 – 46,XX,der(1)t(1;6) (p36.32~36.1;q27)del(1)(p36.33p36.32~36.31),der(6)t(1;6) (p36.32~36.31;q27) – resulting in duplication of chromosomal region 1p36 and deletion of 6qter locus (Figure 2A). The breakpoints of her unbalanced translocation were subsequently determined by chromosomal microarray (CMA): arr[GRCh37] 1p36.31 (5942747_6162645)x3, 6q27 (169685649_170898549) x1 – duplication 1p36.31 (0.22 Mb) and deletion 6q27 (1.2 Mb), and both copy number variations (CNVs) were interpreted as variants of uncertain significance.
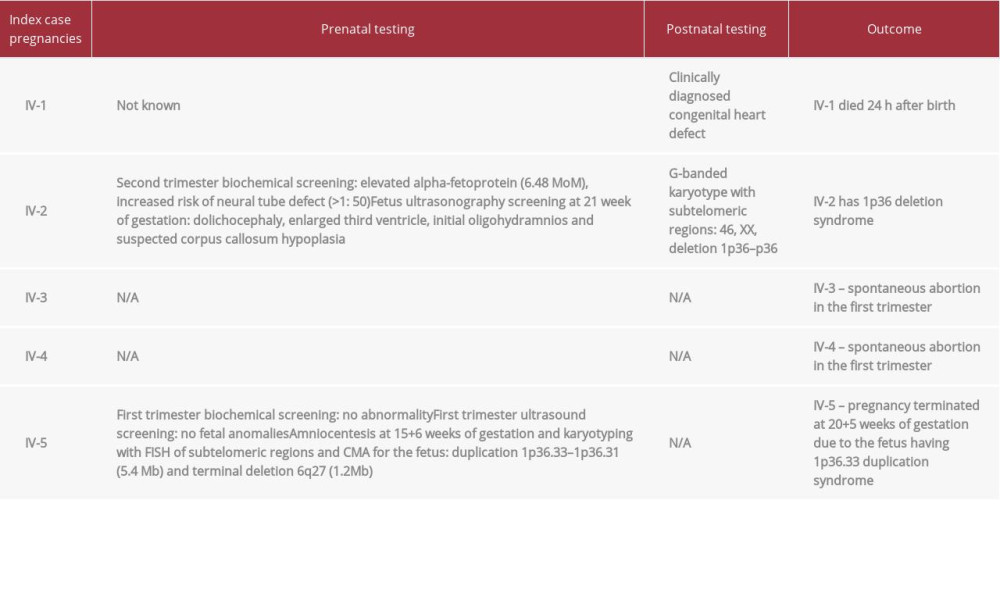
IV-1 (2007). The first child of the index case was a boy, born at term, birth weight 4100 g, who died at the age of 24 h. The established cause of death was congenital heart defect. Genetic testing was not done for this patient.
IV-2 (2015). The index case was referred to our clinic at 21+5 weeks of gestation due to her previous adverse pregnancy outcome and an abnormal second trimester biochemical screening result: elevated alpha-fetoprotein. Ultrasonography revealed multiple fetal developmental anomalies (Table 1). A TORCH screen of serum for infections was negative. The woman refused any invasive prenatal testing. At 23+2 weeks of gestation, ultrasonography showed fetal dolichocephaly, enlarged third ventricle, suspected corpus callosum hypoplasia, and oligohydramnios.
The child, a girl, was born at term, birth weight 3210 g, height 52 cm, Apgar score 7/9. Following birth, she presented with tachypnoea and dilated cardiomyopathy was detected. By the age of 2 months, she developed epileptic seizures. At the same age, corpus callosum hypoplasia was confirmed. She had several dysmorphic features: brachycephaly, high forehead, low hairline, low-set and posteriorly rotated ears, microstomia, and short arms and legs. Genetic testing of IV-2 revealed an abnormal female karyotype with 1p36 deletion.
IV-2 is currently 5 years old. She has severe intellectual disability (with no interest in toys or in communication with others), her speech is unrecognizable, and she cannot walk. MRI of her brain has revealed severe pathological findings: lateral ventricular enlargement, third ventricle enlargement, periventricular demyelination, and a small-sized corpus callosum. Electroencephalogram shows progression and generalization of epileptiform activity, hypsarrhythmia. She has epileptic seizures once a day (usually around 6: 00 am) and receives antiepileptic polytherapy (vigabatrin, topiramate, and levetiracetam). Regarding her cardiovascular system, she has undergone patent ductus arteriosus surgical closure and currently has dilated cardiomyopathy and heart failure II/III (New York Heart Association).
Pregnancies IV-3 and IV-4 were in 2018 and 2019, respectively, and ended spontaneously in the first trimester.
During pregnancy IV-5 (2020), the index case visited our clinic at the 7th/8th week of gestation. Because of high-risk pregnancy and empirical recurrence risk >10%, she was offered CMA for herself and her fetus (Table 1). At 15+6 weeks of gestation, amniocentesis was performed and CMA of amniotic fluid was carried out. Fetal CMA results were: arr[GRCh] 1p36.33p36.31 (752566_6162645)x3 (5.4Mb)-pathogenic; 6q27 (169685649_170898549)x1 (1.2 Mb) – variant of uncertain significance. These results prompted confusion due to a seemingly unstable breakpoint of the inherited CNVs and enlargement of the duplication in the second generation. To define the structure of the chromosomal aberration, we performed karyotyping combined with FISH analysis for chromosomes 1 and 6. The duplicated region was found to be localized at the telomeric end of the long arm of chromosome 6 (Figure 2B). The karyotype analysis allowed us to uncover the nature of the aberration, revealing an unbalanced translocation with a stable breakpoint in both generations. Taking into consideration the expected phenotype of the newborn and the high risk of perinatal death, the patient decided to terminate the pregnancy at 20+1 weeks of gestation and the procedure was carried out 4 days later.
Theoretically, there are 4 possible outcomes for any pregnancy of an unbalanced translocation carrier. Figure 3 schematically illustrates the possible segregations of the first and sixth chromosomes in the index case’s gametes: normal karyotype, the same unbalanced karyotype as that of the index case, and a more pronounced imbalance of chromosomal material that might result in a child with a high likelihood of learning disability with or without congenital anomalies or even spontaneous pregnancy loss (miscarriage). Although normal chromosomal segregation is theoretically possible for the index case, considering index case’s obstetric medical history, gynecologist consultation should be given with specific mention of possible in vitro fertilization technique with preimplantation embryonal testing for structural chromosomal rearrangements (PGT-sr) or long-lasting forms of contraception and permanent sterilization technics.
Discussion
Individuals with unbalanced structural abnormalities have monosomies and/or trisomies of chromosomal segments that can be unique to a particular family. These changes are defined as CNVs. CNVs might be recurrent (ie, with the same size, the same breakpoints and connected to a low copy repeat region), or non-recurrent (ie, of different sizes and breakpoints). CNVs may occur in gametes as unbalanced events caused by low copy repeat-mediated non-homologous recombination and can be passed on to subsequent generations with different consequences.
Patients with unbalanced rearrangements usually have an abnormal phenotype, which is dependent on the nature of the rearrangement, but frequently involves some degree of neuro-developmental disturbances with or without congenital anomalies. However, some cases do not display a specific recognizable clinical phenotype. This may be due to the absence of critical dosage sensitivity genes in the involved chromosomal region. The density of genes in subtelomeric regions is high and even small terminal deletions can lead to a severe disorder.
Since the publication of “Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies” in 2010, CMA is becoming increasingly popular in clinical practice [11]. The success rate of CMA in comparison to G-banded karyotyping is significantly higher and it improves the diagnostic yield in early pregnancy [12]. CMA is often used as the first-line investigation and karyotyping is reserved for special indications. Indications for CMA include multiple congenital abnormalities, the presence of dysmorphic features, unexplained mental retardation, and neurodevelopmental disorders [11]. Indications for karyotyping are infertility, recurrent miscarriages, unexplained stillbirth, malignancy, and chromo-some breakage syndromes.
The index case qualified for comprehensive prenatal genetic testing due to her complicated obstetric medical history and high susceptibility for genetic disorder. In 2015, the first cyto-genetic diagnostic test performed was karyotyping with FISH analysis of the del22q11.2 locus; however, no underlying pathology was detected. At that time, pioneering cytogenetic testing such as CMA was not accessible for routine diagnostics in our clinic. Although carrying out CMA initially would have shortened the path to diagnosis, it would not have uncovered the chromosomal structure crucial for correct risk assessment of future pregnancies and family planning, thereby highlighting the need for a more comprehensive investigation. Accordingly, karyotyping with FISH of certain chromosomal regions, indicated by CMA, revealed the index case’s unbalanced translocation from the first to the sixth chromosome with a concurrent duplication of a small region in the first chromosome (1p36.31).
Conclusions
We would like to emphasize that the technological differences between the cytogenetic and molecular methods make them pertinent to different clinical applications. Furthermore, it is important to emphasize that each method – conventional karyotyping and CMA – should be used appropriately in accordance with the indications. CMA detects differences in the number of DNA copies but does not identify complex structural rearrangements. The choice of investigational method lies with the clinician: either use the one most appropriate for the given clinical situation or use both in a complementary manner.
Figures
References:
1.. Bhatta S, Medows M, Acharya Y, Isolated chromosome 6q27 terminal deletion syndrome: Cureus, 2020; 12(5); 10-14
2.. Heilstedt HA, Ballif BC, Howard LA, Population data suggest that deletions of 1p36 are a relatively common chromosome abnormality: Clin Genet, 2003; 64(4); 310-16
3.. Gajecka M, Mackay KL, Shaffer LG, Monosomy 1p36 deletion syndrome: Am J Med Genet C Semin Med Genet, 2007; 145(4); 346-56
4.. Page SL, Shaffer LG, Nonhomologous Robertsonian translocations form predominantly during female meiosis: Nat Genet, 1997; 15(3); 231-32
5.. Wu Y-Q, Heilstedt HA, Bedell JA, Molecular refinement of the 1p36 deletion syndrome reveals size diversity and a preponderance of maternally derived deletions: Hum Mol Genet, 1999; 8(2); 313-21
6.. Rocha CF, Vasques RB, Santos SR, Paiva CLA, Monosomy 1p36 syndrome: Reviewing the correlation between deletion sizes and phenotypes: Genet Mol Res, 2016; 15(1); gmr.15017942
7.. Jordan VK, Zaveri HP, Scott DA, 1p36 deletion syndrome: An update: Appl Clin Genet, 2015; 8; 189-200
8.. Guterman S, Beneteau C, Redon S, Prenatal findings in 1p36 deletion syndrome: New cases and a literature review: Prenat Diagn, 2019; 39(10); 871-82
9.. El Waly B, Mignon-Ravix C, Cacciagli P, Molecular characterization of a 1p36 chromosomal duplication and in utero interference define ENO1 as a candidate gene for polymicrogyria: Eur J Hum Genet, 2020; 28(12); 1703-13
10.. Marquet V, Bourthoumieu S, Dobrescu A, Familial 1p36.3 microduplication resulting from a 1p-9q non-reciprocal translocation: Eur J Med Genet, 2017; 60(11); 583-88
11.. Miller DT, Adam MP, Aradhya S, Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies: Am J Hum Genet, 2010; 86(5); 749-64
12.. Pauta M, Grande M, Rodriguez-Revenga L, Added value of chromosomal microarray analysis over karyotyping in early pregnancy loss: Systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2018; 51(4); 453-62
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250