13 October 2023: Articles 
Improved Surgical Outcomes in Adults with Congenital Heart Disease and Decompensated Heart Failure: The Role of Perioperative Medical Optimization
Challenging differential diagnosis, Unusual setting of medical care, Congenital defects / diseases
Anton MinaevDOI: 10.12659/AJCR.939230
Am J Case Rep 2023; 24:e939230
Abstract
BACKGROUND: Decompensated heart failure (HF) is recognized as a significant prognostic factor for unfavorable outcomes in both the general population and adults with congenital heart diseases (ACHD). Among ACHD patients, those with advanced heart failure may be candidates for heart transplantation. However, in ACHD patients requiring heart surgery, even with reduced ejection fraction, the administration of appropriate medications can result in improved circulatory parameters, functional class, and surgical outcomes.
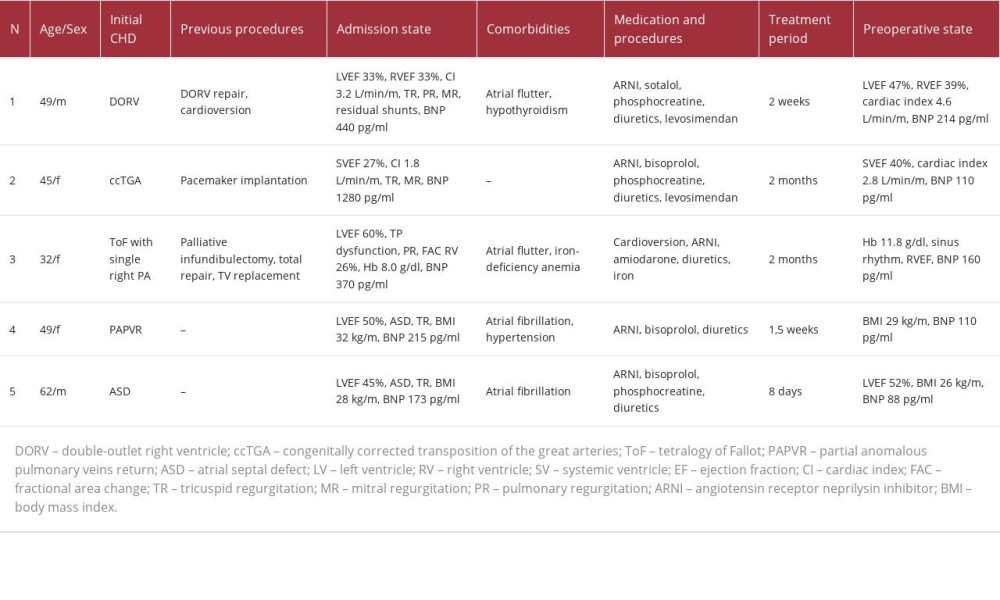
CASE REPORT: We present 5 patients who exhibited indications for open-heart surgery, advanced heart failure (HF) accompanied by congestion, and significant physical activity limitations or symptoms at rest (NYHA class III-IV). Among the patients, 40% were male, with a mean age of 47.4 years (ranging from 32 to 62 years). Three patients displayed reduced systemic ventricular ejection fraction, while 4 patients experienced arrhythmia. Congenital heart diseases (CHD) observed in the patients included repaired double-outlet right ventricle, congenitally corrected transposition of the great arteries, repaired tetralogy of Fallot, partial anomalous right pulmonary venous return, and atrial septal defect. Comprehensive heart failure medications were administered, including an angiotensin receptor neprilysin inhibitor, levosimendan, beta-blockers, phosphocreatine, and diuretics. The preoperative period ranged from 8 days to 2 months. Notably, significant clinical and hemodynamic improvements were observed in all cases, and all open-heart surgeries were successfully completed.
CONCLUSIONS: Advanced and decompensated HF has a high impact on surgical outcomes. Preoperative care with prescribed medical management for ACHD patients is possible and provides good early and mid-term postoperative results.
Keywords: Atrial Septal Defect, Secundum Type, Congenitally Corrected Transposition of the Great Arteries, Heart Failure, Tetralogy of Fallot, Humans, Adult, Male, Middle Aged, Female, Transposition of Great Vessels, Heart Defects, Congenital, Cardiac Surgical Procedures, Ventricular Dysfunction, Left, Treatment Outcome
Background
The emergence and progression of heart failure among adult patients with congenital heart disease is a well-known condition with an incidence rate of 20–45%. Its prevalence in the ACHD population is associated with various functional and hemodynamic features of CHD, arrhythmias, volume or pressure overloads, and myocardial lesions. Heart failure is the leading cause of mortality in this group of patients [1]. Heart transplantation may be considered in patients with advanced heart failure (HF) due to adult congenital heart disease (ACHD) [2]. However, a challenging subgroup consists of patients with decompensated HF and indications for surgical correction. Delayed surgical intervention, along with circulatory disorders and arrhythmias, can exacerbate heart failure progression. On the other hand, decompensated heart failure significantly increases the surgical risk. It is hypothesized that surgery can improve circulation parameters, functional class, and address causes of HF progression without the need for heart transplantation. Nevertheless, the optimal medication strategies for preoperative preparation remain unclear [1,3]. In addition to standard HF therapy (such as renin-angiotensinaldosterone system (RAAS) blockers, beta-blockers, diuretics, and aldosterone antagonists), pharmacological support may include inotropes, antiarrhythmics, and metabolic drugs [4]. This paper highlights the specific aspects of HF correction in ACHD patients undergoing preoperative preparation. The objective of the study was to present a case series of ACHD patients with decompensated HF who have been optimized preoperatively for surgery, and its outcomes.
Case Reports
CASE 1:
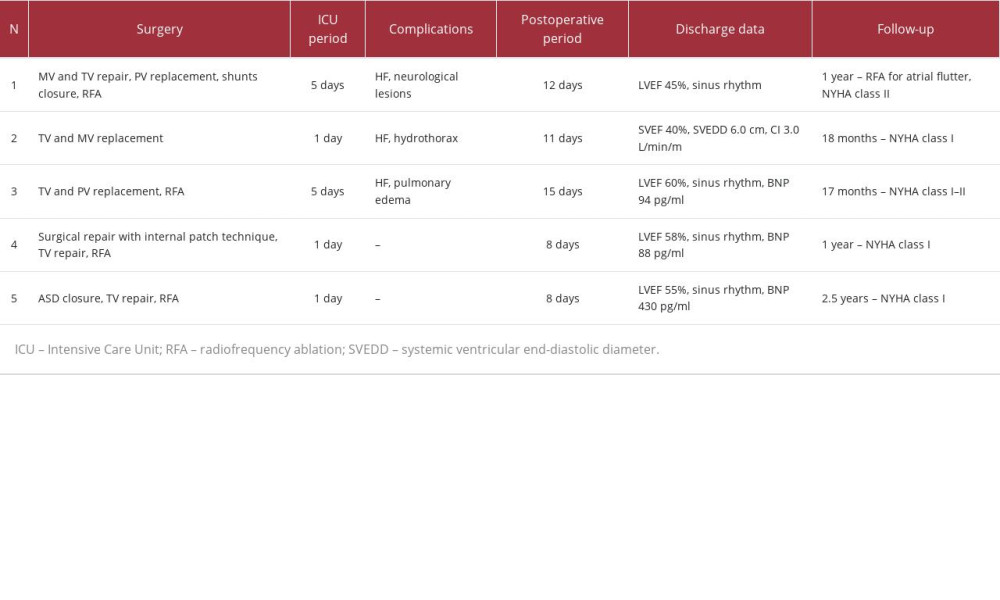
Patient 1 was a 49-year-old man born with double-outlet right ventricle and pulmonary stenosis. He had an anatomic repair with a transannular patch for the right ventricle outflow tract. Upon admission, the patient exhibited peripheral edema, dyspnea, tachycardia, and complex hemodynamic lesions: severe pulmonary and tricuspid regurgitation, moderate mitral regurgitation, residual interventricular and interatrial shunts, severe cardiomegaly, and atrial flutter (with QRS duration up to 160 ms). His BNP level was 440 pg/ml and the creatinine level was 84 μmol/L. Magnetic resonance imaging (MRI) demonstrated reduced left ventricular ejection fraction (LVEF) 33%, right ventricular ejection fraction (RVEF) 33%, cardiac index (CI) 3.2 L/min/m2, right ventricular end-diastolic volume (RVEDV) index 304 ml/m2, and right ventricular end-systolic volume (RVESV) index 205 ml/m2, without signs of significant myocardial fibrosis (Figure 1). Selected medication included valsartan/sacubitril (ARNI, 100 mg twice), sotalol (80 mg twice), phosphocreatine (2 g per day), diuretics (furosemide+spironolactone) and levosimendan (12.5 mg) – 2 doses – at the beginning of treatment and on the eve of surgery. The total preoperative period was 2 weeks. Control preoperative MRI showed increasing of LVEF up to 47%, RVEF 39%, cardiac index 4.6 L/min/m2, RVEDV index 302 ml/m2, and RVESV 184 ml/m2. The BNP level decreased to 214 pg/ml and creatinine was 102 μmol/L. The patient underwent MV and TV repair, pulmonary valve replacement, residual VSD and ASD closure, and right-sided radiofrequency ablation. The patient was extubated after 4 days because of neurological lesions (associated with depressed level of consciousness) and persistent heart failure requiring high doses of vasopressors. Further therapy included valsartan/sacubitril (for 3 months), phosphocreatine, diuretics, and warfarin. The patient was discharged after 12 days. After 1-year follow-up, postoperative paroxysmal atrial flutter was treated by catheter ablation. The patient had mild restrictions of physical activity (NYHA class II).
CASE 2:
Patient 2 was a 45-year-old woman with congenitally corrected transposition of the great arteries. She had only undergone dual-chamber pacemaker implantation for spontaneous AV-block. Her dyspnea, congestion, and exercise intolerance had progressed for a period of 6 months. On admission, echocardiography showed systemic ventricular ejection fraction (SVEF) 27%, systemic ventricular end-diastolic diameter (SVEDD) 6.2 cm, CI 1.8 L/min/m2, TAPSE 11 mm, severe mitral and tricuspid regurgitation, and mild pulmonary hypertension. Positron emission tomography was performed (Figure 2). The BNP level was 1280 pg/ml and the creatinine level was 80 μmol/L. The medications started with valsartan/sacubitril (titrated to 100 mg twice), bisoprolol (2.5 mg), phosphocreatine (2 g per day), and diuretics (torasemide+spironolactone). Levosimendan (12.5 mg) was used once. After 1.5 weeks of treatment, symptoms started to regress and SVEF improved up to 32%. The patient was discharged on oral medication for 2 months. Thereafter, there was an improvement in functional class (according to NYHA), increasing of SVEF to 40%, CI was 2.8 L/min/m2, TAPSE was 16 mm, SVEDD 5.8 cm, BNP was 110 pg/ml, and creatinine was 73 μmol/L. On the eve of surgery, the patient had a second dose of levosimendan (12.5 mg). Tricuspid and mitral valve replacement with mechanical and bio protheses were performed. The patient was extubated after 17 h. Further therapy was continued with valsartan/sacubitril (for 6 months), phosphocreatine, diuretics, and warfarin. On day 11 after the operation, the patient was discharged. After 18 months, she had good exercise tolerance, SVEF was 43%, cardiac index was 3.1 L/min/m2, SVEDD was 5.7 cm, and BNP was 80 pg/ml.
CASE 3:
Patient 3 was a 32-year-old woman born with tetralogy of Fallot with agenesia of the left pulmonary artery, and she underwent palliative RVOT reconstruction and total repair during childhood. At the age of 25 years, tricuspid valve replacement was performed. On admission, the diagnosis consisted of tricuspid bioprosthesis dysfunction (stenosis and insufficiency), severe pulmonary valve regurgitation, and atrial flutter. There was no significant reduction of left ventricular function, but there was for the right ventricle (FAC 26%). Primary laboratory tests showed a BNP level of 370 pg/ml, creatinine 101 μmol/L, and hemoglobin 8.0 g/dl. The right ventricle had severe dilatation (end-systolic volume 92 ml) with an outflow tract diameter up to 31 mm and reduced ejection fraction (Figure 3). Prior medications included diuretics and anticoagulants. The therapy started with amiodarone, valsartan/sacubitril (titrated to 100 mg twice), diuretics (torasemide+spironolactone), and anticoagulants. During the hospital admission, successful cardioversion was performed. After 2 weeks, her BNP level was 83 pg/ml and creatinine was 105 μmol/L. The preoperative period was 1.5 months with the above medications and iron supplements. Prior to surgery, her BNP level was 1604pg/ml, creatinine was 110 μmol/L, and hemoglobin was 11.8 g/dl. The medication was supplemented with phosphocreatine (8 g). The operation consisted of tricuspid bioprosthesis, pulmonary valve replacement, and right-sided radiofrequency ablation. The early postoperative period was complicated with moderate right pulmonary edema, and patient was extubated after 4 days. Further therapy included amiodarone (2 months), valsartan/sacubitril (50 mg twice, 3 months), diuretics, and anticoagulants. At the time of discharge (on day 15 after the operation), her BNP level was 94 pg/ml and creatinine was 93 μmol/L. Twenty months after surgery, she had NYHA class I–II and sinus rhythm.
CASE 4:
Patient 4 was a 49-year-old woman with partial anomalous right pulmonary venous return to the superior vena cava, sinus venosus ASD, severe tricuspid regurgitation, mild mitral regurgitation, and persistent atrial fibrillation (AF). Clinical symptoms started with mild exercise intolerance 10 years before, after the birth of her second child. On admission, she had congestion, dyspnea, and palpitations. Echocardiography showed tricuspid valve annulus dilation with insufficiency, ASD, and LVEF 50%. Catheterization showed mild pulmonary hypertension and intact coronary arteries (Figure 4). Her body mass index (BMI) was 32 kg/m2, BNP was 215 pg/ml, and creatinine was 79 μmol/L. Prescribed medications were valsartan/sacubitril (100 mg twice), bisoprolol (5 mg), diuretics, and anticoagulants. After 1.5 weeks, BMI decreased to 29 kg/m2 and her BNP was 110 pg/ml. Surgical repair with internal pericardial patch baffling, tricuspid annuloplasty, and biatrial radiofrequency ablation were performed. There were no complications, and postoperative therapy consisted of amiodarone, valsartan/sacubitril, diuretics, and warfarin. At discharge 8 days after were surgery, LVEF was 58%, BNP was 88 pg/ml, and she had sinus rhythm. During the 2-year follow-up, there were no cardiac events and she maintained NYHA class I.
CASE 5:
Patient 5 was a 62-year-old man with ASD, moderate tricuspid regurgitation, and persistent AF. Dyspnea and severe exercise intolerance started 4 months before with arrhythmia and progressed quickly. On admission, echocardiography revealed LVEF 45%. ASD without an inferior rim, tricuspid regurgitation with annulus dilatation, and severe cardiomegaly were identified as well (Figure 5). Catheterization showed mild pulmonary hypertension and intact coronary arteries. BMI was 28 kg/m2, BNP was 173 pg/ml, and creatinine was 98 μmol/L. Medications consisted of valsartan/sacubitril (100 mg twice), bisoprolol (2.5 mg), phosphocreatine (2 g per day), diuretics, and anticoagulants. After 8 days, his LVEF increased to 52%, BMI decreased to 26 kg/m2, and BNP was 88 pg/ml. The surgical repair included ASD closure, tricuspid valve repair, and biatrial radiofrequency ablation. There were no significant postoperative HF or arrhythmias, but a high BNP level was noted (up to 430 pg/ml). Postoperative therapy was prescribed with amiodarone, valsartan/sacubitril, diuretics, and warfarin. On day 8 after the operation, he was discharged. During 2.5-year follow-up, no cardiac events developed, and he maintained NYHA class I.
Discussion
Heart failure decompensation is a well-known predictor of adverse outcomes among the general population, and it is the main cause of mortality in ACHD patients, with an incidence rate of 20–45% [1–4]. Event-free survival is 57.5% at 5 years in ACHD patients with HF, compared with 98.0% in ACHD patients without HF [5]. Hemodynamic lesions associated with CHD, such as valvular diseases, chronic volume or pressure overloads, residual shunts, and arrhythmias, can lead to heart failure progression and may require surgical treatment. Preoperative care with an improvement of clinical and hemodynamic state plays an important role in such cases.
The benefits of using RAAS inhibitors have been proven for heart failure treatment in various groups of patients. One of the most recent studies – PARADIGM-HF – aimed to compare valsartan/sacubitril and enalapril. The data of 8442 patients with NYHA class II–IV and reduced LVEF showed a 21% decreased risk of admission and improvement of symptoms [5]. The ACTIVITY-HF study found no significant differences in peak oxygen consumption after 12 weeks between the sacubitril/valsartan and enal-april groups [6]. Appdadurai et al showed good efficacy, tolerability, and adverse effects in the ACHD heart failure population with reduced systemic ventricular ejection fraction (mean 27%). Among the 5 patients, 2 had systemic right ventricles. Previous RAAS inhibitors medications included ACEIs or ARBIs. During a 6-month follow-up period after valsartan/sacubitril initiation, there was improvement in all patients according to the NYHA classification, and partial increase of peak oxygen consumption, but there was no data about ejection fraction and cardiac index [7]. Maurer et al studied outcomes of 23 ACHD patients with reduced ejection fraction: 12 patients had systemic right ventricles, 7 had systemic left ventricles, and 4 had single ventricles. The mean titrated daily dose was 230 mg and the mean follow-up period was 221 days. There was no reliable improvement in the NYHA classification, NT-proBNP level, and systemic ventricle ejection fraction [8]. Zandstra et al studied use of valsartan/sacubitril in 20 patients with systemic right ventricles, demonstrating improvement of systolic function, 6-min walk test results, decreasing of NT-proBNP level, and no significant changes of ejection fraction, with a mean daily dose of ARNI 322 mg [9]. Research shows the potential benefits of the use of ARNI in pre- and postoperative care of ACHD patients with symptomatic heart failure, and even in patients with impaired kidney function [10,11].
Levosimendan use, safety, and efficiency have been shown in studies such as Levo-Rep, LION-Heart, and LAICA [12,13], but use of levosimendan for preoperative care before cardiac surgery is rare. Case series with chronic heart failure using levosimendan infusion in ACHD patients have been published. Cranley et al demonstrated outcomes of 3 patients with repaired CHD: pulmonary atresia, ventricular septal defect, and tetralogy of Fallot. All patients had symptomatic heart failure with reduced ejection fraction from 20% to 43%. Prescribing monthly levosimendan infusion led to reduced symptoms and BNP level and higher exercise tolerance. Also, there was an assumption about benefits for treatment of biventricular heart failure, that was common among ACHD patients [14]. However, it is still unclear what medication was the leading cause of systemic ventricular function improvement in our case series.
Prescription of phosphocreatine for heart failure treatment and myocardial protection is increasingly being used. A decrease in creatine level against the background of chronic heart failure by 33–43% was detected in in vivo experiments. The inhibition of creatine transporter and high ATP consumption were suggested as the main reasons for creatine deficit [15,16], and this was confirmed in research comparing 2 groups – a healthy group of patients and adults with heart failure. The correlation between low creatine level and reduced LV ejection fraction was determined as well [17]. A meta-analysis by Mingxing et al showed that phosphocreatine use in cardiac surgery was associated with reduced rates of intraoperative inotropic support, major arrhythmias, and increased spontaneous recovery of the cardiac rhythm immediately after aortic declamping, as compared to standard treatment [18]. Carvalho et al found no myocardial function improvement after phosphocreatine monotherapy, but there was a clear increase of peak oxygen consumption, which may be associated not only with myocardial metabolism but also with skeletal muscular metabolism improvement [19]. On the other hand, interim research by Tereschenko et al included data of 465 patients with chronic HF NYHA class II–IV, with mildly reduced and significantly reduced LV EF. The use of phosphocreatine in addition to basic therapy was associated with significant improvement of LV EF and 6-min walk test results [20]. In our experience, phosphocreatine was prescribed in patients with reduced LV (SV) EF or right ventricular dysfunction.
In general, the research discussed above demonstrates approaches in the management of heart failure, which developed without surgical causes and patients did not need surgical interventions. The relationship of chronic heart failure before surgery and surgical outcomes was studied in patients with coronary artery disease. Initial heart failure worsened early and late after myocardial revascularization and the worst risk ratio was identified for patients who had symptomatic heart failure with reduced LV ejection fraction [21]. It can be assumed that the same factors, as well as arrhythmias, comorbidities, and multiple hemodynamic lesions, can affect surgical outcomes in ACHD. Also, in our study group, the progression of heart failure was associated with the history of arrhythmia. These pathologies are related and impair each other, as emphasized by rapid deterioration of the clinical picture. The increasing risk of arrhythmias in ACHD is well known and requires timely outpatient reviews [22–25]. Other than that, the role of diuretics is not totally clear. In preoperative treatment, the use of diuretics can result in symptomatic improvement, optimization of fluid and electrolyte imbalance, blood volume, pre-load and afterload, BMI, and organ perfusion, which greatly affect cardiac surgery and postoperative intensive care [26–29].
Conclusions
In our study, patients had heart failure with reduced or preserved LV ejection fraction, and different preoperative treatments and strategies of care were used. Increased cardiac index and improvement of symptoms was associated with optimal medication in all patients with reduced, mildly reduced, and borderline EF. Complete surgery, following optimization, demonstrated favorable early and mid-term outcomes. The use of this drug group appears to be beneficial and is encouraged, and there is a need to explore the potential of new drugs such as SGLT2 inhibitors. Nevertheless, more research on perioperative care in ACHD patients is necessary, preferably in a larger series and potentially through an international registry of ACHD.
Figures
References:
1.. Baumgartner H, De Backer J, Babu-Narayan SV, ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease.: Eur Heart J, 2021; 42(6); 563-645
2.. Mangini S, Pires PV, Braga FG, Bacal F, Decompensated heart failure: Einstein (Sao Paulo), 2013; 11(3); 383-91
3.. Burstein DS, Rossano JW, Griffis H, Greater admissions, mortality and cost of heart failure in adults with congenital heart disease: Heart, 2021; 107; 807-13
4.. McMurry JJV, Packer M, Desai AS, Angiotensin-neprilysin inhibition versus enalapril in heart failure: N Engl J Med, 2014; 371; 993-1005
5.. Maessen L, De Meester P, Troost E, Short-term prognostic value of heart failure diagnosis in a contemporary cohort of patients with adult congenital heart disease: Can J Cardiol, 2023; 39(3); 292-301
6.. Halle M, Schöbel C, Winzer EB, A randomized clinical trial on the short-term effects of 12-week sacubitril/valsartan vs. enalapril on peak oxygen consumption in patients with heart failure with reduced ejection fraction: Results from the ACTIVITY-HF study.: Eur J Heart Fail, 2021; 23(12); 2073-82
7.. Appdadurai V, Thoreau J, Malpas T, Sacubitril/valsartan in adult congenital heart disease patients with chronic heart failure – a single centre case series and a call for an international registry: Heart Lung Circ, 2020; 29(1); 137-41
8.. Maurer SJ, Pujol Salvador C, Schiele S, Sacubitril/valsartan for heart failure in adults with complex congenital heart disease: Int J Cardiol, 2020; 300; 137-40
9.. Zandstra TE, Nederend M, Jongbloed MRM, Sacubitril/valsartan in the treatment of systemic right ventricular failure: Heart, 2021; 107(21); 1725-30
10.. Yan L, Loh JK, Tan JL, Sacubitril/valsartan for heart failure in patients with complex adult congenital heart disease – experience from a tertiary centre in Singapore.: Int J Cardiol Congenit Heart Dis., 2021; 6; 100268
11.. Krishnathasan K, Dimopoulos K, Duncan N, Advanced heart failure in ACHD: The Role of renal dysfunction in management and outcomes.: Eur J Prev Cardiol., 2023 [Online ahead of print]
12.. Comín-Colet J, Manito N, Segovia-Cubero J, Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial.: Eur J Heart Fail, 2018; 20; 1128-36
13.. Altenberger J, Parissis JT, Costard-Jaeckle A, Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: A multicentre randomized trial: Eur J Heart Fail, 2014; 16(8); 898-906
14.. Cranley J, Hardiman A, Freeman LJ, Pulsed Levosimendan in advanced heart failure due to congenital heart disease: A case series: Eur Heart J Case Rep, 2020; 4(3); 1-6
15.. Fox AC, Wikler NS, Reed GE, High energy phosphate compounds in the myocardium during experimental congestive heart failure. Purine and pyrimidine nucleotides, creatine, and creatine phosphate in normal and in failing hearts.: J Clin Investig, 1965; 44; 202-18
16.. Ten Hove M, Chan S, Lygate C, Mechanisms of creatine depletion in chronically failing rat heart: J Mol Cell Cardiol, 2005; 38; 309-13
17.. Nakae I, Mitsunami K, Matsuo S, Myocardial creatine. Concentration in various nonischemic heart diseases assessed by 1H magnetic resonance spectroscopy.: Circ. J, 2005; 69; 711-16
18.. Mingxing F, Landoni G, Zangrillo A, Phosphocreatine in cardiac surgery patients: A meta-analysis of randomized controlled trials: J Cardiothorac Vasc Anesth, 2018; 32(2); 762-70
19.. Carvalho APPF, Rassi S, Fontana KE, Influence of creatine supplementation on the functional capacity of patients with heart failure: Arq Bras Cardiol, 2012; 99; 623-29
20.. Tereschenko SN, Perepech NB, Cheremisina IA, Interim results of the BYHEART observational study: Exogenous phosphocreatine effect on the quality of life of patients with chronic heart failure: Kardiologiia, 2021; 61(7); 22-27
21.. Dalén M, Lund LH, Ivert T, Holzmann MJ, Sartipy U, Survival after coronary artery bypass grafting in patients with preoperative heart failure and preserved vs reduced ejection fraction: JAMA Cardiol, 2016; 1(5); 530-38
22.. Anter E, Jessup M, Callans DJ, Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic: Circulation, 2009; 119(18); 2516-25
23.. Budts W, Roos-Hesselink J, Rädle-Hurst T, Treatment of heart failure in adult congenital heart disease: A position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology: Eur Heart J, 2016; 37(18); 1419-27
24.. Hindricks G, Potpara T, Dagres N, ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC: Eur Heart J, 2021; 42(5); 373-498
25.. Richards M, Di Somma S, Mueller C, Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: Results from the BACH Study (Biomarkers in ACute Heart Failure): JACC Heart Fail, 2013; 1(3); 192-99
26.. Kendsersky P, Krasuski RA, Intensive Care Unit management of the adult with congenital heart disease: Curr Cardiol Rep, 2020; 22; 136
27.. Agha RA, Franchi T, Sohrabi C, Mathew G, The SCARE 2020 guideline: Updating Consensus Surgical CAse REport (SCARE) guidelines.: Int J Surg, 2020; 84; 226-30
28.. Verzelloni Sef A, Sef D, Garcia Saez D, Heart transplantation in adult congenital heart disease with the organ care system use: A 4-year single-center experience: ASAIO J, 2021; 67(8); 862-68
29.. Verzelloni Sef A, Jaggar SI, Trkulja V, Factors associated with long-term outcomes in adult congenital heart disease patients with infective endocarditis: A 16-year tertiary single-centre experience.: Eur J Cardiothorac Surg., 2023; 63(5) ezad105
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250