29 August 2023: Articles 
Co-Presentation of Lupus Nephritis with Autoimmune Hepatitis
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Rare coexistence of disease or pathology
Fizah S. Chaudhary1ABDEF, Amit SureenDOI: 10.12659/AJCR.940478
Am J Case Rep 2023; 24:e940478
Abstract
BACKGROUND: Systemic lupus erythematosus (SLE) is a multiorgan immunologic disease which commonly results in systemic manifestations by involving joints, kidneys, skin, heart, hematologic cell lines, pulmonary and central nervous systems. The hepatic involvement of lupus is relatively less common, which creates diagnostic challenges, as the clinical presentations of lupus hepatitis and autoimmune hepatitis (AIH) are similar.
CASE REPORT: A 51-year-old woman presented for multiple joint pain that began 2 years ago. Her work-up, including kidney biopsy, was consistent with a diagnosis of class V lupus nephritis. Subsequently, within a few months, she was admitted with acute elevation of liver enzymes and high immunoglobulin IgG level, and a liver biopsy demonstrated impressive interface hepatitis with many plasma cells and lymphocytes, suggestive of chronic hepatitis with high histological activity. This case illustrates the co-presentation of lupus nephritis and AIH, which is a rare association. The patient was managed with a tapering dose of prednisone, hydroxychloroquine initially, and later with mycophenolate mofetil, with complete resolution of liver enzyme abnormalities by 4-month follow-up.
CONCLUSIONS: Lupus hepatitis is hepatic involvement of SLE, which should be distinguished from AIH. Accurate diagnosis is important, as management and prognosis of these immunologic conditions can differ. Although both entities share clinical and biochemical markers, the presence of anti-ribosomal P antibodies and liver histology features of predominant lymphoid infiltrates with lobular inflammation favor lupus hepatitis. A multidisciplinary approach involving rheumatologists, hepatologists, and pathologists can improve disease outcomes by properly differentiating the 2 entities and guiding the selection of appropriate immunosuppressive therapy.
Keywords: Autoimmune Diseases, Biopsy, Liver Diseases, Lupus Erythematosus, Systemic, Female, Humans, Middle Aged, lupus nephritis, Hepatitis, Autoimmune, Kidney
Background
Systemic lupus erythematosus (SLE) is a multiorgan autoimmune disease, which commonly involves joints, kidneys, skin, heart, hematologic cell lines, lungs, and the central nervous system. Autoimmune hepatitis (AIH) is an immunogenic disorder of the liver characterized by chronic liver inflammation due to autoantibodies, predominantly anti-smooth antibody (ASMA) and immunoglobulin IgG. Extrahepatic manifestations of AIH such as autoimmune thyroiditis, rheumatoid arthritis, and SLE result from common immune-mediated pathways [1,2]. Hepatic involvement of SLE occurs in 50–60% of individuals during the spectrum of their disease [3,4]. Overlapping syndrome of SLE and AIH is an entity distinct from lupus hepatitis that requires exclusion of other causes of elevated liver enzymes such as viral hepatitis, drug-induced liver injury, non-alcoholic fatty liver disease, alcohol-associated liver disease, hemochromatosis, Wilson disease, and other autoimmune liver diseases, including primary biliary cholangitis and primary sclerosing cholangitis. Both SLE and AIH can present with common clinical presentations such as arthralgia, positive antinuclear antibodies (ANA), and elevated immunoglobulin IgG level. Anti-double-stranded DNA (anti-dsDNA) is considered highly specific to SLE, but can also be positive in patients with AIH [5,6]. Similarly, ASMA is positive in 99% of patients with SLE [7]. The overlap of clinical and diagnostic modalities poses challenges in establishing diagnosis and management of patients with overlapping syndrome of SLE and AIH. We present a case of 51-year-old woman who initially presented with inflammatory arthritis and lupus nephritis, and subsequently developed autoimmune hepatitis.
Case Report
A 51-Year-old African American woman with past medical history notable only for breast cancer presented to the Rheumatology clinic for evaluation of multiple joint pain for 2 years. She reported fatigue, Raynaud phenomenon, and worsening pain in her knuckles, wrists, hands, knees, and feet bilaterally. She smoked half a pack of cigarettes per day, reported having 1–2 drinks of alcohol per week, and denied recreational drugs abuse, recent change in her medications, or herbal supplements use. Her physical examination was normal. Laboratory work-up was notable for mild elevation of alanine aminotransferase (ALT) 67 (10–35) U/L, aspartate aminotransferase (AST) 46 (0–32) U/L, alkaline phosphatase (ALP) 149 (35–104) u/L, creatinine 1.60 (0.5–1.0) mg/dL, positive antinuclear antibody (ANA) with titer 1: 1280, anti-ribonucleoprotein (RNP) >643 (<20) RLU, elevated C-reactive protein (CRP) 1.24 (0–0.50) mg/dL, ESR 43 (0–30) mm/H, rheumatoid factor (RF) antibody IgM 5.1 (<4.9), negative RF IgA, and anti-cyclic citrullinated peptide (CCP) <4.6 (<19.9) RLU. Complement levels were normal, including C3 level 103 (83–193) mg/dL and C4 level 26 (15–57) mg/dL. Urinalysis revealed proteinuria 100 mg/dL, spot urine protein/creatinine ratio ~1: 1 (72/73.5) without RBC. SLE was diagnosed and the patient was referred for kidney biopsy. The kidney biopsy findings were consistent with class V lupus nephritis. Treatment was initiated with hydroxychloroquine (HCQ) 200 mg twice daily, prednisone 10 mg daily, and mycophenolate mofetil (MMF) 500 mg twice daily. She was lost to follow-up and was hospitalized 3 months later with jaundice, generalized pruritus, and acute elevation of liver enzymes, including ALT 418, AST 302, ALP 641, total bilirubin (TB) 6.40 (0.0–1.6) mg/dL, and elevated creatinine 1.55 mg/dL. Her additional work-up was notable for elevated immunoglobulin IgG level1980 (767–1590) mg/dL. Serologies were negative for acute viral hepatitis, including hepatitis A antibodies IgM, hepatitis B surface antigen, hepatitis B core antibodies IgM, and hepatitis C antibodies by ELISA. Abdominal CT scan and MRCP were unremarkable except for non-specific borderline enlarged retroperitoneal lymph nodes. The liver biopsy demonstrated markedly distorted hepatic architecture by dense and mixed infiltrates involving the portal and periportal areas, lobular parenchyma, focal bridging fibrosis (stage 3/4 fibrosis), and impressive interface hepatitis with many plasma cells and lymphocytes suggestive of chronic hepatitis with high histological activity (Figures 1–4). HCQ and MMF were held, and she was discharged on oral prednisone 40 mg once daily. After 4 months of treatment with high-dose steroids (prednisone 40 mg daily), the liver enzymes normalized. Hepatology considered starting azathioprine (AZA) for AIH, which historically has been the preferred steroid-sparing agent for AIH. However, her proteinuria persisted, and Rheumatology considered azathioprine would be a suboptimal choice to treat lupus nephritis. After discussing the options, Hepatology and Rheumatology services agreed MMF may be suitable to treat both AIH and lupus nephritis. She was restarted on hydroxychloroquine 200 mg twice daily and MMF 1.5 g twice daily.
Discussion
Overlapping syndrome of SLE and AIH is an uncommon entity that was first reported in 1950. There is an overlap of clinical presentation, laboratory markers, and histology that makes it difficult to distinguish weather hepatic involvement is due to SLE, known as lupus hepatitis, or due to AIH, which are 2 separate disorders.
Systemic lupus erythematosus is a systemic autoimmune condition that predominantly affects middle-aged women (female-to-male ratio 10: 1) with incidence rate of 0.3–31.5 cases per 100 000 persons yearly [8]. Environmental factors such as smoking, exposure to ultraviolet radiation, drugs, and familial aggregation of genetic variations in T cell signaling pathways are leading mechanisms in pathogenesis of the disease. These mechanisms result in production of lupus-specific autoantibodies and deposition of immune complexes in various organs such as skin, joints, kidneys, central nervous system, heart, lungs, and blood vessels, which results in systemic manifestations of SLE [8]. European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria are 98% sensitive and 96% specific for establishing the diagnosis of SLE. Our patient fulfilled the EULAR/ACR classification criteria of SLE, including entry criteria (positive ANA with titer of 1: 1280), joint involvements (+6), and renal biopsy class V lupus nephritis (+8), with an aggregate score of 14 [9].
Autoimmune hepatitis is also an immune-mediated chronic inflammatory condition of the liver that predominantly affects females (71–95%) ages 10–30 and 40–60 years [10]. Autoreactivity of CD4 and CD8 T cells with concurrent lack of effective B regulatory cell inhibition results in production of B cell autoreactive antibodies that play a fundamental role in the pathogenesis of liver inflammation and hepatic fibrosis [10]. It is characterized by transaminitis, elevated immunoglobulin IgG levels, presence of certain antibodies such as antinuclear antibody (ANA) (80%), anti-smooth muscle antibody (ASMA) (63%), liver kidney microsomes type 1 (LKM1) (3%), soluble liver antigen (SLA) (7–22%), and histological hallmark features of interface hepatitis with predominant plasma cells (60%), lobular hepatitis (47%), and centrilobular necrosis (29%) [10,11]. The revised International Autoimmune Hepatitis Group (IAIHG) scoring system may be used to establish diagnosis in cases of atypical presentation when diagnosis remains uncertain [12]. Our patient fulfilled the revised IAIHG criteria of AIH with total pretreatment score of a minimum of 18 (normal <15) based on the following scores: female (+2), ALP: AST or ALP: ALT ratio 1.5–3.0 (0), serum IgG 1980 (+1),ANA 1280 (+3), negative viral hepatitis (+3), no recent or current use of hepatotoxic drugs (+1), ethanol intake <25 g/day (+2), liver histology positive for interface hepatitis (+3), predominant lymphoplasmacytic infiltrate (+1), and presence of other autoimmune disease (+2). The simplified diagnostic criteria are an additional tool to establish the diagnosis of AIH, with specificity of 100% [13]. A simplified score of ≥7 has a sensitivity of 87.1% (95% confidence interval (CI); 84.5–87.6), specificity of 99.6% (95% CI: 98.2–99.9), positive likelihood ratio of 229 (95% CI 46–1294) and negative likelihood ratio of 0.13 (95% CI: 0.12–0.15) [13]. Our patient also fulfilled the simplified criteria for AIH with a score of 8 based on the following scores: ANA titer ≥1: 80 (+2), IgG level >1.1 times of upper limit of normal (+2), serologies negative for viral hepatitis (+2), and liver histology typical of AIH (+2).
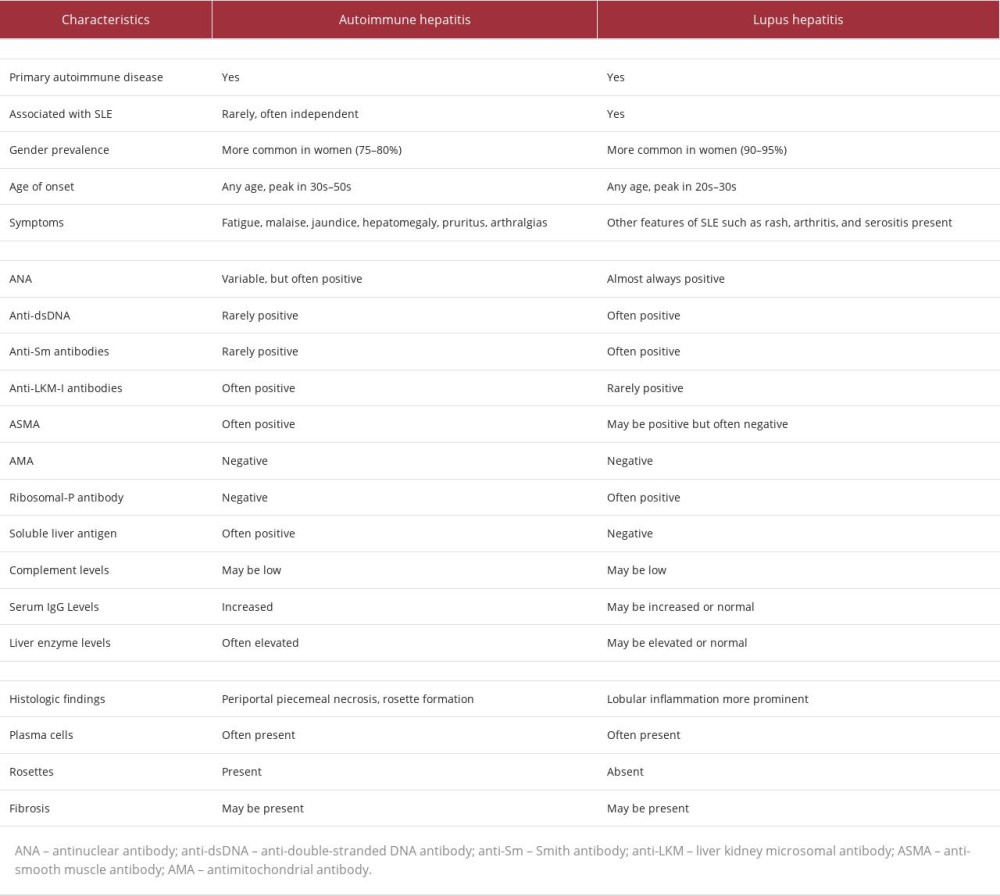
Hepatic involvement associated with SLE (lupus hepatitis) and AIH are considered 2 separate entities, but both autoimmune diseases share common clinical features, including polyarthralgia, and serological markers such as transaminitis, positive ANA, ASMA, anti-RNP, anti-cardiolipin antibodies, and high IgG globulins[14]. Patients with AIH are at increased risks of having connective tissue diseases and vice versa [15]. To add to the confusion of clinicians, AIH has been historically called “lupoid hepatitis” by some experts [4]. The presence of anti-ribosomal P antibodies has been reported to be more specific for the diagnosis of lupus hepatitis and it is typically absent in AIH [4,16]. Liver biopsy is the preferred diagnostic modality to establish the etiology of hepatic inflammation [16]. The presence of interface hepatitis is the hallmark AIH, but presence of lymphoid infiltrates with lobular inflammation is suggestive of immune-mediated hepatic involvement of SLE [16,17]. However, liver biopsy findings should not be interpreted in isolation but rather in the clinical context since there may be overlapping features. Taken together, clinical presentation, serologies, pathologic findings, and EULAR/ACR criteria are useful tools to establish a diagnosis and distinction of AIH from lupus hepatitis (Table 1). Our patient fulfilled EULAR/ACR criteria of SLE and revised IAIHG criteria of AIH. The final diagnosis of our patient was overlapping syndrome of SLE and AIH.
Corticosteroids therapy is the mainstay treatment of overlapping SLE and AIH syndrome. Once clinical and biochemical remission is achieved, corticosteroids should be switched to steroid-sparing immunosuppressant agents such as AZA or MMF (in some cases) to maintain remission [18,19]. In case of recurrent relapses, long-term use of low-dose steroids combined with AZA is recommended to achieve sustained remission. Our patient was treated with steroids to treat AIH and then was switched to MMF. Mycophenolate mofetil is preferred as the most effective treatment of lupus nephritis and as second-line treatment for AIH [20]. The overall prognosis in untreated patients with overlapping SLE and AIH is poor, with a 5-year survival rate of only 25%, but timely treatment with combination therapy improves survival to 80% [4].
Conclusions
Lupus hepatitis is hepatic involvement of SLE, which should be distinguished from AIH. Although both entities share clinical and biochemical markers, the presence of anti-ribosomal P antibodies and liver histology features of predominant lymphoid infiltrates with lobular inflammation favor lupus hepatitis. A high index of clinical suspicion is required for early identification and differentiation of AIH from lupus hepatitis, as the former is a more aggressive disorder and results in high mortality if untreated. Recent literature supporting the use of mycophenolate mofetil to treat AIH is a welcome development, since mycophenolate mofetil can be effectively used to treat several manifestations of SLE, especially lupus nephritis.
Figures
References:
1.. Heneghan MA, Yeoman AD, Verma S, Autoimmune hepatitis: Lancet, 2013; 382(9902); 1433-44
2.. Takahashi A, Rai T, Onizawa M, Autoimmune hepatitis complicated by late-onset systemic lupus erythematosus: Hepatol Res, 2007; 37(9); 771-74
3.. Runyon BA, LaBrecque DR, Anuras S, The spectrum of liver disease in systemic lupus erythematosus. Report of 33 histologically-proved cases and review of the literature: Am J Med, 1980; 69(2); 187-94
4.. Adiga A, Nugent K, Lupus hepatitis and autoimmune hepatitis (lupoid hepatitis): Am J Med Sci, 2017; 353(4); 329-35
5.. Czaja AJ, Morshed SA, Parveen S, Nishioka M, Antibodies to single-stranded and double-stranded DNA in antinuclear antibody-positive type 1-autoimmune hepatitis: Hepatology, 1997; 26(3); 567-72
6.. Beisel C, Weiler-Normann C, Teufel A, Lohse AW, Association of autoimmune hepatitis and systemic lupus erythematodes: A case series and review of the literature: World J Gastroenterol, 2014; 20(35); 12662-67
7.. Rahman A, Isenberg DA, Systemic lupus erythematosus: N Engl J Med, 2008; 358(9); 929-39
8.. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT, Update on the diagnosis and management of systemic lupus erythematosus: Ann Rheum Dis, 2021; 80(1); 14-25
9.. Aringer M, Costenbader K, Daikh D, 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus: Ann Rheum Dis, 2019; 78(9); 1151-59
10.. Mack CL, Adams D, Assis DN, Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases: Hepatology, 2020; 72(2); 671-722
11.. Czaja AJ, Carpenter HA, Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis: Gastroenterology, 1993; 105(6); 1824-32
12.. Chandok N, Silveira MG, Lindor KD, Comparing the simplified and international autoimmune hepatitis group criteria in primary sclerosing cholangitis: Gastroenterol Hepatol (N Y), 2010; 6(2); 108-12
13.. Muratori P, Granito A, Pappas G, Muratori L, Validation of simplified diagnostic criteria for autoimmune hepatitis in Italian patients: Hepatology, 2009; 49(5); 1782-83 author reply 1783
14.. Leggett BA, The liver in systemic lupus erythematosus: J Gastroenterol Hepatol, 1993; 8(1); 84-88
15.. West M, Jasin HE, Medhekar S, The development of connective tissue diseases in patients with autoimmune hepatitis: A case series: Semin Arthritis Rheum, 2006; 35(6); 344-48
16.. Lohse AW, Rolls Royce for everybody? Diagnosing liver disease by mini-laparoscopy: J Hepatol, 2011; 54(3); 584-85
17.. Usta Y, Gurakan F, Akcoren Z, Ozen S, An overlap syndrome involving autoimmune hepatitis and systemic lupus erythematosus in childhood: World J Gastroenterol, 2007; 13(19); 2764-67
18.. Alvarez F, Ciocca M, Cañero-Velasco C, Short-term cyclosporine induces a remission of autoimmune hepatitis in children: J Hepatol, 1999; 30(2); 222-27
19.. Salem C ME, Murr TEI, Case report and review of literature: An overlap syndrome of autoimmune hepatitis and systemic lupus erythematous: Clin Med Rev Case Rep, 2019; 6(1); 249-52
20.. Santiago P, Schwartz I, Tamariz L, Levy C, Systematic review with meta-analysis: Mycophenolate mofetil as a second-line therapy for autoimmune hepatitis: Aliment Pharmacol Ther, 2019; 49(7); 830-39
Figures
In Press
26 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943893
27 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942126
27 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943098
27 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943725
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250