14 December 2023: Articles 
A 51-Year-Old Woman with Advanced Peritoneal Mesothelioma and Stage 3b Chronic Kidney Disease Treated with Cytoreductive Surgery and Bidirectional Intraperitoneal Cisplatin and Ifosfamide Chemotherapy: A Case Report
Unusual clinical course, Unusual setting of medical care
Marwan Alaswad1ADEF*, Fadwa ElKordy1DE, Heba Jaamour1EF, Abdullah Al Otry1DE, Ayman Z. Azzam23ABDE, Tarek M. Amin3AD, Hatim A. Khoja45DEDOI: 10.12659/AJCR.941726
Am J Case Rep 2023; 24:e941726
Abstract
BACKGROUND: Malignant mesotheliomas are rare, yet highly malignant tumors. Mesotheliomas are tumors that develop from mesothelial surfaces, with the pleura being the most common, followed by the peritoneum. The diagnosis of malignant peritoneal mesothelioma (MPM) is usually established when the disease is advanced, owing to the nonspecific clinical appearance and abdominal symptoms. Initially, MPM was treated with palliative systemic chemotherapy, with or without palliative surgery. However, cytoreductive surgery (CRS) combined with bidirectional intraoperative chemotherapy (BDIC) has recently emerged as a treatment option for MPM. BDIC creates a bidirectional chemotherapy gradient in the peritoneal tumor cells through the simultaneous use of intraperitoneal and intravenous chemotherapy. CRS, combined with BDIC (CRS-BDIC), allows the complete elimination of residual tiny tumor cells after complete removal of the visible tumor nodules.
CASE REPORT: Herein, we present a case of a 51-year-old woman with MPM and chronic kidney disease (CKD) stage 3b. Her treatment consisted of neoadjuvant chemotherapy and immunotherapy, followed by CRS-BDIC using intraperitoneal cisplatin and doxorubicin, and intravenous ifosfamide. The surgery was successful, with no immediate complications or decline in the patient’s kidney function. On follow up 2 months later, the patient denies suffering any chemotherapy-related adverse effects, and her kidney profile remains stable.
CONCLUSIONS: In conclusion, nephrotoxicity, a known adverse effect of cisplatin and ifosfamide, might not be a contraindication for the use of these potentially nephrotoxic drugs in CRS-BDIC in patients with renal impairment.
Keywords: Cytoreduction Surgical Procedures, hyperthermic intraperitoneal chemotherapy, Mesothelioma, Malignant, Peritoneal Neoplasms, Renal Insufficiency, Chronic
Background
Malignant mesotheliomas are rare, yet highly malignant tumors. A mesothelioma is a tumor that develops from mesothelial surfaces, such as the pleura (65–70%), peritoneum (30%), tunica vaginalis, testis, and pericardium (1–2% combined) [1]. Because of the extended latent time between start and symptoms, as well as the common and nonspecific clinical appearance, peritoneal and pleural mesotheliomas are frequently misdiagnosed [2]. Patients with MPM most commonly present with nonspecific abdominal symptoms such as abdominal pain (33%) or abdominal swelling (31%) [3], and this usually leads to diagnosis when the condition is advanced [2]. The most significant risk factor for MPM is exposure to asbestos; other risk factors include exposure to radiation, and SV40 virus [4]. The diagnosis of MPM cannot be made based solely on imaging findings because the findings are often unspecific and insufficient [5]. The definitive diagnosis of MPM relies upon histopathologic examination of the resected specimen, along with immunohistochemical examination, employing markers such as calretinin [5].
There are few choices for treating MPM. The initial treatment for these cases was palliative systemic chemotherapy, either alone or combined with palliative surgery, with an average survival of less than 7 months [6]. Hyperthermic intraperitoneal chemotherapy (HIPEC), defined as a locoregional therapy that entails the intraoperative chemoperfusion of heated chemo-therapy to eliminate residual tiny tumor cells following significant excision of visible tumor nodules, is used in the modern treatment of MPM [7]. Numerous studies found that CRS combined with HIPEC had better survival rates compared to palliative surgery combined with systemic chemotherapy in terms of patient survival [8], with a meta-analysis revealing a predicted 5-year survival rate of 42% in MPM patients treated with CRS plus HIPEC [9]. These encouraging results of CRS combined with HIPEC led doctors to treat MPM with CRS followed by HIPEC and intraoperative intravenous (iv) ifosfamide [10]. Chemotherapeutic drugs injected intravenously (iv) penetrate the tumor nodules’ capillaries to the residual illness. A bidirectional chemotherapy gradient in the peritoneal tumor cells is possible when intravenous and intraperitoneal (ip) chemotherapy is given simultaneously [11].
Cytoreductive surgery with bidirectional intraperitoneal chemotherapy (CRS-BDIC) is an aggressive therapy with morbidity and death risks using iv ifosfamide and ip cisplatin and doxorubicin [10]. Nephrotoxicity is one of the known non-surgical adverse effects that can be triggered using cisplatin and ifosfamide; therefore, readjusting the dose of cisplatin or ifosfamide given is mandatory in patients with abnormal kidney profile [12].
Case Report
A 51-year-old woman, known to have stage 3b CKD, was referred to our hospital as a case of MPM for management and treatment. Two months ago, in another institution, she presented with epigastric pain, and a CT scan showed peritoneal thickening associated with omental caking and ascites. A biopsy was done and confirmed the diagnosis of MPM.
CT done in our institution revealed similar findings to the prior CT (Figure 1). Subsequently, PET-CT revealed diffuse peritoneal thickening with multiple areas of FDG activity, compatible with the diagnosis of MPM (Figure 2).
Based on the imaging results, the tumor was deemed inoperable at this time, and we decided to proceed with neoadjuvant chemotherapy. Her first cycle consisted of a combination of cisplatin and pemetrexed. Her condition was complicated by nausea, febrile neutropenia, thrombocytopenia, bacteremia, acute kidney injury, and mucositis, all thought to be cisplatin-induced. She was started on vancomycin and piperacillintazobactam for febrile neutropenia and on iv fluids for her AKI.
For the remaining chemotherapy cycles, cisplatin was switched to carboplatin to reduce her severe adverse effects. After her last cycle of chemotherapy, she was febrile again and not tolerating oral administration. She had recurrent, severe febrile neutropenia, nausea, and severe mucositis. Her AKI persisted and progressed, with her creatinine level reaching 162 umol/l. This mandated starting continuous renal replacement therapy (CRRT), along with iv fluids. Her AKI started to improve slowly after receiving CRRT, eventually reaching a stable creatinine baseline of 140 (umol/L).
The patient could not tolerate any more chemotherapy due to the severe complications that she developed and this mandated withholding any further chemotherapy. Subsequently, she started immunotherapy with pembrolizumab. After completing the pembrolizumab course, PET-CT showed complete resolution of the previously noted mild FDG-avid peritoneal mesothelioma as well as ascites, with only a residual tiny (6 mm) focal ill-defined soft-tissue lesion seen at the right superior omentum at the hepatic flexure level. No significant FDG-avid disease was seen elsewhere (Figure 3).
Her preoperative eGFR was 35 ml/min/m2 and her creatinine level was 139 umol/L (Table 1). We decided to proceed with CRS-BDIC using 50% intraperitoneal cisplatin with a dose of 25 mg/m2 plus doxorubicin with a dose of 7.5 mg/m2 infused over 90 minutes, simultaneously with 80% intravenous ifosfamide dose of 1040 mg/m2 and a mesna dose of 208 mg/m2 (1/5 of the iv ifosfamide dose). Both intraperitoneal and intravenous chemotherapy were started simultaneously after completing exploratory laparotomy with peritonectomy, omen-tectomy, splenectomy, appendectomy, hysterectomy, and bilateral salpingo-oophorectomy. Intraoperatively, continuous monitoring of her mean arterial pressure (MAP), systolic blood pressure (SBP), blood loss and transfusions, and urine output was done (Table 2). A continuous renal replacement (CRRT) machine was present and ready to be used throughout the entire length of the operation in case the patient developed nephrotoxicity (due to either of the drugs given) or became overloaded. Continuous monitoring of her urine output was done throughout the entire operation and especially every 15 minutes of the BDIC phase, with her intraoperative urine output ranging between 2.5 and 3 ml/kg/h. The procedure went well, without any immediate complications. The patient was able to maintain satisfactory blood pressure and did not require any inotropes or vasopressors. Continuous monitoring of her renal function by a nephrologist was done closely during and after the surgery, with her renal function remaining stable without the need of either intraoperative or postoperative hemodialysis (Table 1). Post-operatively, histopathologic examination of the resected tissue samples was performed and showed an infiltrative malignant neoplasm with cytologic atypia, numerous cell mitoses, and papillary architecture composed of sheets of epithelioid cells that stained diffusely positive for calretinin (mesothelial marker) and were negative for BerEp4 (an epithelial marker), with absent gland formation, squamous differentiation, and mucin production, confirming the diagnosis of MPM (Figure 4A–4C).
Her postoperative course was uncomplicated, she was stable on close monitoring in the ICU, and did not develop any chemotherapy-related adverse effects. On follow-up 2 months later, her kidney function remained stable, and her serum creati-nine was 109 umol/L and eGFR of 44 ml/min/m2.
Discussion
Cisplatin is a chemotherapy drug that is used in the treatment of multiple solid-organ malignancies [13]. Cisplatin has been associated with many toxicities, including ototoxicity, gastrotoxicity, and myelosuppression, but the main dose-limiting adverse effect of cisplatin is nephrotoxicity [14,15]. Cisplatin nephrotoxicity can present in a variety of different ways, but one of the most serious and more common presentations is acute kidney injury (AKI), which occurs in 20–30% of patients receiving cisplatin [16]. Other presentations include hypomagnesemia [17] and chronic kidney disease (CKD) [18].
Nephrotoxicity associated with the use of cisplatin in HIPEC has been reported to occur in 3.7% to 36% of cases [12]. In a retrospective study of 113 patients who received cisplatin-based chemotherapy as the primary intervention, new-onset CKD stage 3 developed in 19 (12.1%) patients, with this increase being directly proportionate to the dose and number of cycles given [19].
Cashin et al studied the pharmacokinetics of cisplatin use in HIPEC and found that 46% of cisplatin was absorbed into the plasma from the peritoneal cavity when 50 mg/m2 cisplatin is given ip [20]. They also concluded that lowering the perfusion time to 60 minutes does not significantly affect the pharmacokinetics of cisplatin and warrants consideration [20].
Aggressive hydration using isotonic fluid remains the main method for lowering the risk of cisplatin-induced nephrotoxicity, nevertheless; the risk of cisplatin nephrotoxicity is still not eliminated even with aggressive hydration [21]. The incidence of cisplatin-induced nephrotoxicity was 50% prior to the induction of hydration regimens [22], but after the introduction of the hydration regimens, the incidence rate fell to 3.7% [23]. Morgan et al performed a retrospective study examining the effectiveness of forced diuresis with mannitol combined with hydration in the reduction of cisplatin-induced nephrotoxicity [24]. A 2.6-fold increased risk of cisplatin-induced nephrotoxicity (
Ifosfamide demonstrated heat synergy in pharmacokinetic studies and was found in tumor nodules when administered continuously in BDIC [10]. Kintzel et al advises lowering the dose of ifosfamide given by 25% in patients with creatinine clearance of 45 ml/min and a reduction of 30% in patients with creatinine clearance of 30 ml/min [25]. In a prospective study of 18 patients who underwent BDIC with intravenous ifosfamide, 6 patients (33.3%) developed ifosfamide-induced nephrotoxicity [26].
We have previously highlighted the successful use of CRS-BDIC using intraperitoneal cisplatin and doxorubicin, with intravenous ifosfamide and mesna in a patient with stage 5 CKD [27]. This case report highlights the second successful case managed in our institution using the same technique and regimen, in a patient with stage 3b CKD.
Conclusions
In conclusion, nephrotoxicity, a known adverse effect of cisplatin and ifosfamide, might not be a contraindication for the use of potentially nephrotoxic drugs in CRS-BDIC in patients with renal failure, as highlighted by the 2 cases managed in our institution. Both patients received the same regimen, and their kidney function remained stable. However, further studies are required to achieve the best level of care possible for this group of patients.
Figures
References:
1.. Raptopoulos V, Peritoneal mesothelioma: Crit Rev Diagn Imaging, 1985; 24(4); 293-328
2.. Li CY, Alexander HR, Peritoneal metastases from malignant mesothelioma: Surg Oncol Clin N Am, 2018; 27(3); 539-49
3.. Acherman YIZ, Welch LS, Bromley CM, Sugarbaker PH, Clinical presentation of peritoneal mesothelioma: Tumori, 2003; 89(3); 269-73
4.. Antman K, Hassan R, Eisner M, Update on malignant mesothelioma: Oncology (Williston Park), 2005; 19(10); 1301-9 ; discussion 1309–10, 1313–16
5.. Bridda A, Padoan I, Mencarelli R, Frego M, Peritoneal mesothelioma: A review: MedGenMed, 2007; 9(2); 32
6.. Chahinian AP, Pajak TF, Holland JF, Diffuse malignant mesothelioma. Prospective evaluation of 69 patients: Ann Intern Med, 1982; 96(6 Pt 1); 746-55
7.. González-Moreno S, González-Bayón LA, Ortega-Pérez G, Hyperthermic intraperitoneal chemotherapy: Rationale and technique: World J Gastrointest Oncol, 2010; 2(2); 68-75
8.. Passot G, Vaudoyer D, Villeneuve L, What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1,125 procedures: J Surg Oncol, 2016; 113(7); 796-803
9.. Helm JH, Miura JT, Glenn JA, Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: A systematic review and meta-analysis: Ann Surg Oncol, 2015; 22(5); 1686-93
10.. Van der Speeten K, Stuart OA, Pharmacokinetic study of perioperative intravenous Ifosfamide: Int J Surg Oncol, 2011; 2011; 185092
11.. de Bree E, Michelakis D, Stamatiou D, Pharmacological principles of intraperitoneal and bidirectional chemotherapy: Pleura Peritoneum, 2017; 2(2); 47-62
12.. Ye J, Ren Y, Wei Z, Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: A retrospective cohort study: Surg Oncol, 2018; 27(3); 456-61
13.. Hartmann JT, Lipp HP, Toxicity of platinum compounds: Expert Opin Pharmacother, 2003; 4(6); 889-901
14.. Sastry J, Kellie SJ, Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine: Pediatr Hematol Oncol, 2005; 22(5); 441-45
15.. Arany I, Safirstein RL, Cisplatin nephrotoxicity: Semin Nephrol, 2003; 23(5); 460-64
16.. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB, Mechanisms of Cisplatin nephrotoxicity: Toxins (Basel), 2010; 2(11); 2490-518
17.. Sutton RA, Walker VR, Halabe A, Chronic hypomagnesemia caused by cisplatin: Effect of calcitriol: J Lab Clin Med, 1991; 117(1); 40-43
18.. Brillet G, Deray G, Jacquiaud C, Long-term renal effect of cisplatin in man: Am J Nephrol, 1994; 14(2); 81-84
19.. Suer E, Mermerkaya M, Gülpınar Ö, Does the number of cycles of cisplatin based chemotherapy have any effect on renal function in patients with testicular germ cell tumor?: J Urol, 2013; 190(6); 2081-85
20.. Cashin PH, Ehrsson H, Wallin I, Pharmacokinetics of cisplatin during hyperthermic intraperitoneal treatment of peritoneal carcinomatosis: Eur J Clin Pharmacol, 2013; 69(3); 533-40
21.. Crona DJ, Faso A, Nishijima TF, A systematic review of strategies to prevent cisplatin-induced nephrotoxicity: Oncologist, 2017; 22(5); 609-19
22.. Launay-Vacher V, Rey JB, Isnard-Bagnis C, Prevention of cisplatin nephrotoxicity: State of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care: Cancer Chemother Pharmacol, 2008; 61(6); 903-9
23.. Hakeam HA, Breakiet M, Azzam A, The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery: Ren Fail, 2014; 36(10); 1486-91
24.. Morgan KP, Snavely AC, Wind LS, Rates of renal toxicity in cancer patients receiving cisplatin with and without mannitol: Ann Pharmacother, 2014; 48(7); 863-69
25.. Kintzel PE, Dorr RT, Anticancer drug renal toxicity and elimination: Dosing guidelines for altered renal function: Cancer Treat Rev, 1995; 21(1); 33-64
26.. Hakeam H, Ayman A, Waleed AT, Amen T, Systemic complications of the bidirectional intraoperative chemotherapy with intravenous ifosfamide and hyperthermic intraperitoneal chemotherapy (HIPEC) using cisplatin plus doxorubicin: Pleura Peritoneum, 2019; 4(4); 20190025
27.. Almesned R, Azzam AZ, Aldeheshi A, Amin TM, Bidirectional intraoperative chemotherapy using cisplatin and ifosfamide for intraperitoneal mesothelioma in severe renal impairment: A case report: Am J Case Rep, 2023; 24; e938192
Figures
Tables
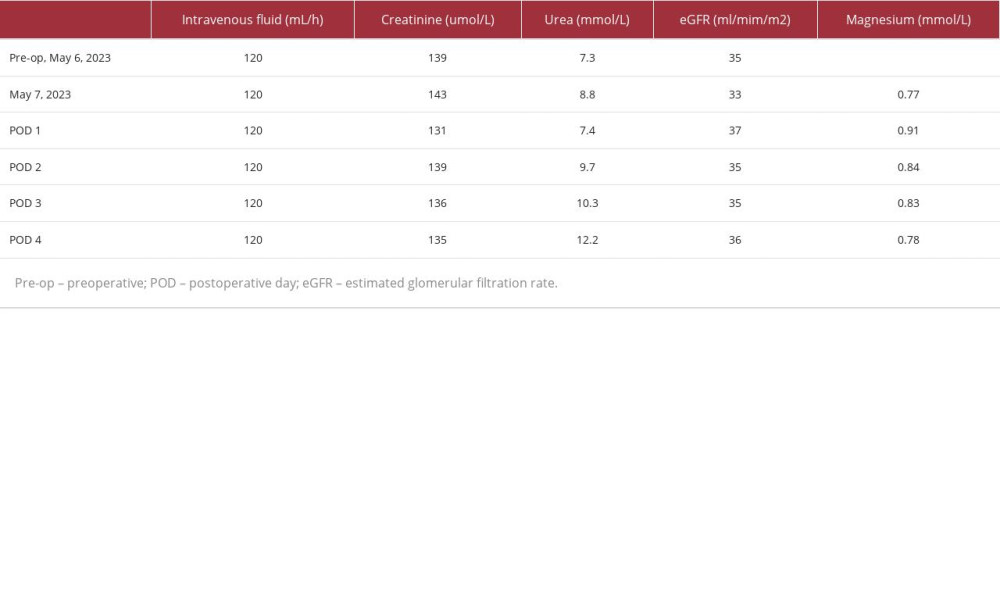 Table 1.. Preoperative and postoperative renal profile values, intravenous fluids administered, and Magnesium levels.
Table 1.. Preoperative and postoperative renal profile values, intravenous fluids administered, and Magnesium levels.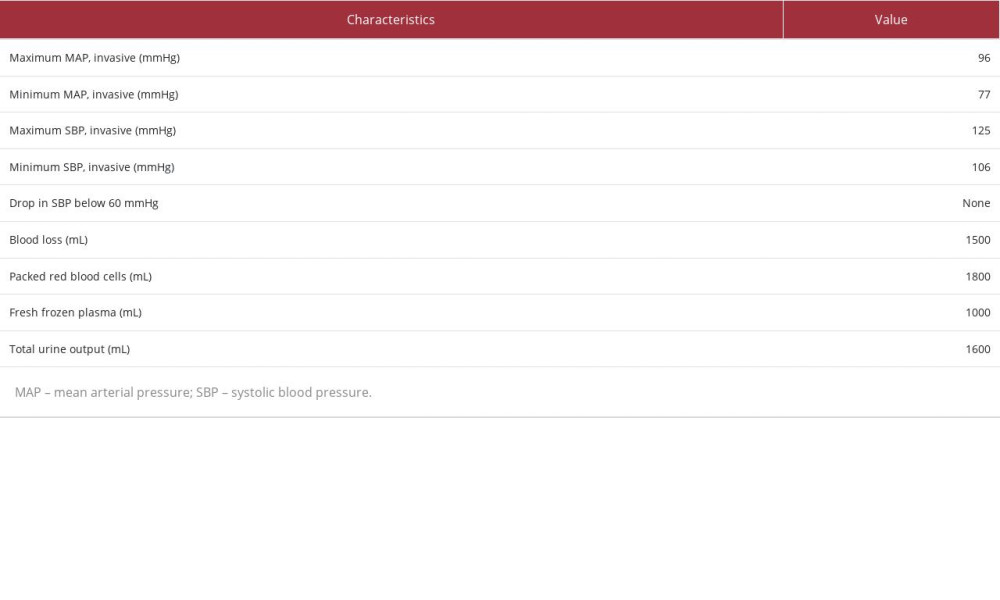 Table 2.. Intraoperative values of the monitored parameters.
Table 2.. Intraoperative values of the monitored parameters. Table 1.. Preoperative and postoperative renal profile values, intravenous fluids administered, and Magnesium levels.
Table 1.. Preoperative and postoperative renal profile values, intravenous fluids administered, and Magnesium levels. Table 2.. Intraoperative values of the monitored parameters.
Table 2.. Intraoperative values of the monitored parameters. In Press
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942921
22 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943346
24 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943560
26 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943893
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








