17 January 2024: Articles 
Successful Treatment of Systemic Light Chain Amyloidosis with Liver Involvement using Low-Frequency Daratumumab: A Case Report
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Unexpected drug reaction, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Xinyi Meng1CEF, Jingsong He1BC, Fei Cheng2BC, Hui Yan3BD, Chunting Zhu1DF, Xing Guo1DF, Yi Li1AB, Zhen Cai1AG, Donghua He1A*DOI: 10.12659/AJCR.942534
Am J Case Rep 2024; 25:e942534
Abstract
BACKGROUND: Systemic light chain (AL) amyloidosis is a disease characterized by the deposition of amyloid fibrils throughout tissues due to the production of misfolded immunoglobulin light chains by clonally expanded populations of CD38+ plasma cells. Some patients can have liver involvement, which typically presents with nonspecific symptoms. Daratumumab, a human CD38-targeting antibody, has shown efficacy in improving hematological parameters and organ function in patients with AL amyloidosis. Low-frequency daratumumab can reduce financial burden, but whether it is effective for patients with liver involvement has not been reported.
CASE REPORT: We present the case of a 64-year-old man admitted to our hospital with fatigue and recurrent fever. Histological analysis of a liver biopsy demonstrated AL amyloidosis. Bone marrow biopsy demonstrated the presence of abnormal plasma cells. Laboratory test results demonstrated increased levels of circulating free kappa (κ) light chains, which were also seen on blood and urine immunofixation electrophoresis. Based on these findings, AL amyloidosis of the κ light chain type with liver, cardiac, and renal involvement was diagnosed. The patient ultimately achieved hematological stringent complete response, liver remission, renal complete response, and cardiac very good partial response after 2 cycles of the low-frequency daratumumab, bortezomib, and dexamethasone regimen and 4 cycles of daratumumab and dexamethasone regimen chemotherapy.
CONCLUSIONS: The case indicates that low-frequency daratumumab treatment can have efficacy in AL amyloidosis with liver involvement.
Keywords: Daratumumab, Immunoglobulin Light-chain Amyloidosis
Background
Systemic light chain (AL) amyloidosis is a hematological disorder arising from clonal expansion of plasma cells that produce misfolded immunoglobulin light chains. These light chains can then form amyloid fibrils that deposit in tissues and organs, causing progressive structural damage and organ dys-function [1]. The annual incidence of AL amyloidosis in China is approximately 10 to 14 cases per million [2]. Only 20% to 30% of patients have clinical evidence of liver involvement [3,4]; however, the prognosis of AL amyloidosis with liver involvement is poor, with a median survival time of 8.5 months [5]. Patients with elevated bilirubin levels have a median survival of only 4 months [6]. The cyclophosphamide, bortezomib, and dexamethasone (CyBorD) regimen is the commonly used treatment for AL amyloidosis currently. Daratumumab directly targets CD38 and has direct anti-tumor and immunomodulatory effects [7,8]. The Andromeda study reported that the combination of daratumumab with CyBorD resulted in hematologic response in 91.8%, complete hematologic response in 53.3%, cardiac response in 41.5%, and renal response in 53% of patients after 6 months of treatment [9]; however, the proportion of patients achieving liver response was not reported.
Herein, we report the case of a 64-year-old man who presented with fatigue and recurrent fever and was treated with low-frequency daratumumab for AL amyloidosis as part of a prospective clinical study (clinical trial registration number ChiCTR2100049253) to investigate the efficacy of daratumumab for AL amyloidosis with liver involvement. Low-frequency daratumumab was administered at 16 mg/kg every 2 weeks for weeks 1 through 8 and every 4 weeks for weeks 9 through 24, either alone or in combination with bortezomib. The regimen can be discontinued after week 25, or daratumumab mono-therapy may be continued every 8 weeks for up to 2 years for maintenance therapy.
Case Report
A 64-year-old male patient was admitted to the hospital with a 1-month history of fatigue and a 2-week history of recurrent fever, with acute viral hepatitis initially suspected. He had a history of hypertension. He described a recent weight loss of 15 kg. The patient’s Eastern Cooperative Oncology Group score was 2. On physical examination, jaundice, scleral icterus, and a slightly distended abdomen were observed; however, the liver and spleen were not palpable. Biochemical tests demonstrated high levels of total and direct bilirubin (299.8 μmol/L and 246.3 μmol/L, respectively; reference range 0–26.0 μmol/L and 0–8.0 μmol/L, respectively), with raised glutamicpyruvic transaminase (132 U/L; reference range 9–50 U/L) and alkaline phosphatase (ALP, 405 U/L; reference range 45–125 U/L) levels. Serum hepatitis B core antibody testing was positive. Serum levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) (552 pg/mL; reference range 0–486 pg/mL), BNP (170.2 pg/mL; reference range 0–121 pg/mL), and troponin I (0.07 ng/mL; reference range 0–0.034 ng/mL) were also elevated. Serum creatinine level was 69 μmol/L (reference range 57–111 μmol/L), glomerular filtration rate was 94 mL/ min, with urine protein 2+ (1.0 g/L). No significant abnormalities were observed in blood routine tests, coagulation parameters, and cardiac enzymes.
Abdominal computed tomography (CT) and liver magnetic resonance imaging (MRI) revealed multiple low-density shadows in the liver, possibly indicative of infectious lesions and pelvic and abdominal effusion. Ultrasound-guided liver puncture biopsy was performed on September 10, 2021, with pathological analysis demonstrating acute liver injury. Immunohistochemical analyses were as follows: hepatitis B surface antigen (−), CK19 (bile duct +), CK7 (bile duct +), MUM1 (small amount +), PAS (+), D-PAS (−), masson (+), reticular fiber staining (+), iron staining (−), copper staining (−), EBER (−), Congo red (±), lappa (κ) (+), and lambda (λ) (−).
A massive amount of red-stained amorphous material was deposited in the hepatic sinusoidal space, which was considered indicative of amyloidosis (Figures 1–4). Blood and urine immunofixation electrophoresis demonstrated positive IgG and κ free light chains (FLCs). Serum FLC measurements revealed an elevated κ light chain concentration of 55.3 mg/L (reference range 6.7–22.4 mg/L) with a ratio of 3.12. The urinary concentrations of κ light chain and λ light chain were 41.3 mg/dL (reference range 0–0.7 mg/dL) and 10.5 mg/dL (reference range 0–0.4mg/dL), respectively. Routine bone marrow biopsy demonstrated an abnormal plasma cell population comprising approximately 0.27% of the total population and mild hematopoietic tissue hyperplasia. The patient subsequently received 3 treatments with artificial liver support systems (September 07, 2021, September 09, 2021, and September 13, 2021), leading to significant relief of nausea and fatigue and a reduction in the total bilirubin level to 164.9 μmol/L. The patient was transferred to our Hematology Department for treatment on September 27, 2021, with laboratory test results demonstrating negative FISH analysis of bone marrow and 100% IgKC on mass spectrometry (Figure 5).
In order to distinguish it from transthyretin amyloidosis, TTR gene mutation was detected, and the result was negative. The serum M protein level was 3.3 g/L. Elevated total protein and M protein (11999 mg/24 h and 1667.9 mg/L, respectively) were observed on 24-h urine testing. Cardiac ultrasound and MRI indicated symmetrical thickening of the left ventricular wall (septal thickness of approximately 15 mm), decreased left ventricular diastolic function, and possible myocardial amyloidosis. AL amyloidosis of κ light chain type with liver, cardiac, and renal involvement was diagnosed. The Mayo 2004 stage was stage II, the Mayo 2012 stage was stage I, and renal staging was stage II.
The patient received the first cycle of daratumumab, bortezomib, and dexamethasone (DVD) regimen chemotherapy (daratumumab 16 mg/kg every 2 weeks, bortezomib 2.0 mg days 1, 4, 8, and 11, and dexamethasone 20 mg days 1, 2, 4, 5, 8, 9, 11, and 12) on October 02, 2021. Considering the poor liver function and weakness of the patient, cyclophosphamide was not added, and bortezomib and dexamethasone were also reduced. The chemotherapy resulted in a notable reduction in the size of the inhomogeneous low-density liver lesions, compared with the initial abdominal CT study.
After the first round of chemotherapy, the difference between involved and uninvolved FLCs (dFLC) decreased to less than 10 mg/L, and the urine protein level decreased markedly to 554 mg/24 h. However, serum ALP (453 U/L) levels were largely unchanged, and serum NT-proBNP levels increased by 30% to >300 pg/mL. Accordingly, treatment responses were classified as follows: hematological, very good partial response; liver, stable disease; renal, partial response; and cardiac, progressive disease.
The second cycle of DVD chemotherapy (daratumumab 1100 mg every 2 weeks, bortezomib 2.3 mg days 1 and 4; 2.0 mg day 8, and dexamethasone 20 mg on days 1, 2, 4, 5, 8, and 9) was administered on November 04, 2021, during which the patient had adverse effects including infection, abdominal distension, diarrhea, edema, and hypokalemia. Serum BNP levels increased from 152.9 pg/mL to 640.9 pg/mL, and serum NT-proBNP levels increased from 1163 pg/mL to 3328 pg/mL. These findings were considered to represent combined immunologic injury on the basis of impaired cardiac function after multidisciplinary collaborative diagnosis and treatment.
The third round of daratumumab and dexamethasone (DD) chemotherapy (daratumumab 1100 mg every 4 weeks, and dexamethasone 20 mg on days 1, 2, 4, 5, 8, and 9) was administered on December 03, 2021, with 500 mg/day methylprednisolone shock therapy for 6 days. After the third round of chemotherapy, treatment responses were classified as follows: hemato-logical response, stringent complete response; liver, stable disease; renal, very good partial response; cardiac, partial response.
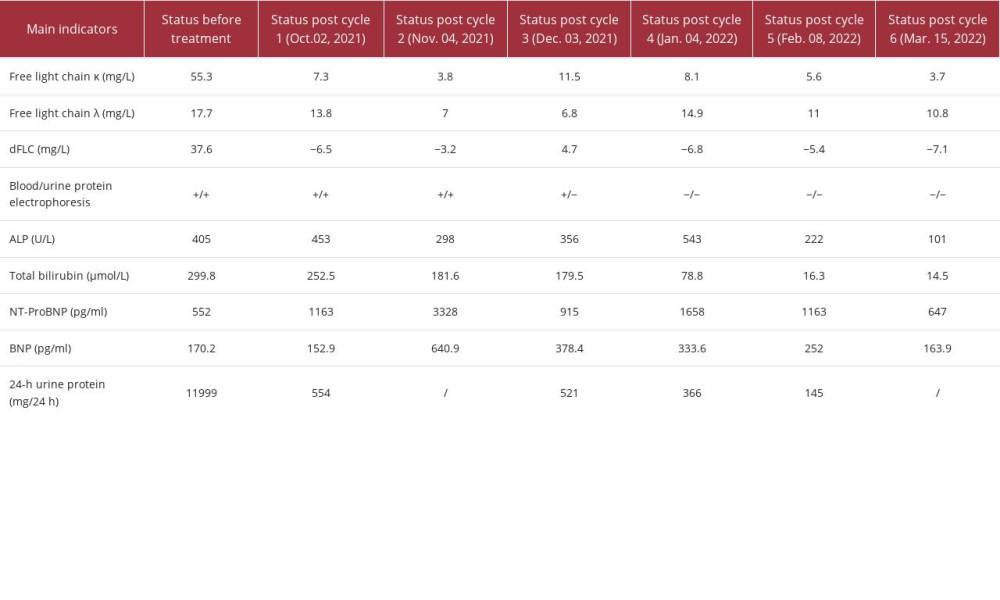
After the fourth through sixth cycles of DD chemotherapy (daratumumab 1000 mg every 4 weeks, and dexamethasone 20 mg on days 1, 2, 4, 5, 8, and 9) from January 04, 2022, February 08, 2022, and March 15, 2022, laboratory test results demonstrated negative blood and urine immunofixation electrophoresis, normal serum FLC κ and λ levels, a >50% decrease in serum ALP levels, from a maximum of 543 U/L to 101 U/L, a decrease in urinary protein from 11999 mg/24 h to 145 mg/24 h, and a >60% decrease in serum NT-proBNP levels. Treatment responses were classified as follows: hematological, stringent complete response; liver, remission; renal, complete response; and cardiac, very good partial response. Table 1 shows changes in serum and urine parameters during the 6 cycles of chemotherapy. The patient was treated with the DD regimen for maintenance. As of November 2023, the patient was alive, and the hematological and organ responses were maintained.
Discussion
AL amyloidosis is a disease derived from plasma cell clones, characterized by extracellular deposition of amorphous amyloid fibers derived from the N-terminus of the monoclonal light chain variable region [10]. The most common organs involved are the heart and kidney, while other affected organs include the liver, autonomic or peripheral nerves, digestive tract, and soft tissues of the skin [11]. Although histological evidence of liver involvement was reportedly observed in approximately 70% of cases with systemic amyloidosis in a previous study [12], only 20% to 30% of patients have clinical evidence of liver involvement. Amyloidosis confined to the liver alone is uncommon. In a study of 102 cases of AL amyloidosis with liver involvement conducted in China, 25.5% of cases had involvement of 2 organs and 31.4% had involvement of 3 organs, including heart and kidney involvement in 71.6% and 68.6% of cases, respectively [6].
Liver involvement often manifests as fatigue, poor appetite, weight loss, abdominal pain, ascites, and hepatomegaly. Other rare complications include jaundice, spontaneous liver rupture, and liver failure [13,14]. Laboratory examinations typically demonstrate markedly increased serum levels of ALP, accompanied by mildly abnormal liver function and mildly increased serum bilirubin and transaminase levels. Imaging typically demonstrates findings similar to those seen in fatty liver disease, such as liver enlargement and inhomogeneous densities within the liver. Patients with liver involvement have a poor prognosis, with 5-year and 10-year survival rates reported as 13.0% to 16.9% and 1.0% to 6.6%, respectively [5,15].
As the symptoms of AL amyloidosis are often complex and nonspecific, early identification is essential to improve the poor prognosis of AL amyloidosis. Therefore, clinicians should also suspect the possibility of amyloidosis in addition to more common liver diseases in patients with fatigue, hepatic discomfort, and weight loss, particularly those with hepatic enlargement or elevated serum ALP levels. Accordingly, tissue and bone marrow biopsies should be performed as soon as possible when amyloidosis is highly suspected, with pathological examination invariably demonstrating positive Congo red staining in cases of AL amyloidosis [16,17]. Relevant gene testing should also be conducted in cases of suspected hereditary amyloidosis.
The principle of AL amyloidosis treatment is targeted elimination of monoclonal plasma cells and inhibition of precursor proteins to reduce the deposition of amyloid material. The CyBorD regimen (bortezomib, cyclophosphamide, and dexamethasone) or VD regimen (bortezomib, and dexamethasone) are now commonly used clinically. The Andromeda study demonstrated that the addition of the anti-CD38 antibody, daratumumab, to the CyBorD regimen was associated with higher frequencies of hematologic complete response, survival free from major organ deterioration or hematologic progression, and cardiac and renal response at 6 months [9]. Daratumumab has a rapid onset, with a median time to hematological response of 1 week [18]. In 28 patients during the introductory phase of the Andromeda study, the overall organ response rate was 64%. Organ response was observed in 2 of the 4 patients with liver involvement (50%), with a median time to response of 330 days [19]. A case of AL amyloidosis confined to the liver and bone marrow in the absence of significant serum light chain elevation was reported, with enhanced hematologic and organ responses after 3 cycles of CyBorD and 5 cycles of daratumumab with CyBorD [16]. The Mayo Clinic reported a case of advanced cardiac and hepatic AL amyloidosis in which serum FLC levels normalized after daratumumab administration for the first time [20]. However, there is still a lack of substantial clinical data and statistical evidence regarding the efficacy and safety of daratumumab in cases of AL amyloidosis with liver involvement.
At present, the dose and frequency of daratumumab for AL amyloidosis are similar to the treatment regimen used for multiple myeloma. However, considering the substantially lower number of CD38+ plasma cells and tumor load in patients with AL amyloidosis compared with that of patients with multiple myeloma and the economic burden of daratumumab [21], it is of practical significance to determine the appropriate dose and frequency of daratumumab for patients. Thus, we adopted a low-frequency daratumumab regimen (registration number ChiCTR2100049253) of every 2 weeks in cycles 1 and 2, every 4 weeks in cycles 3 through 6, and every 8 weeks thereafter, until disease progression. In this case, we adopted 2 cycles of the low-frequency DVD regimen (daratumumab, bortezomib, and dexamethasone) and 4 cycles of the DD regimen (daratumumab and dexamethasone) for a total of 6 cycles of chemotherapy. We then evaluated treatment response after each cycle of treatment according to the validated hematologic response criteria for AL amyloidosis [22], including hematological and organ responses. The first cycle of chemotherapy achieved a reduction of dFLC to <10 mg/L but did not achieve the negative immunofixation electrophoresis necessary for classification as hematologic complete response.
After the first cycle, the 24-h urine protein decreased significantly; in addition to the effectiveness of the treatment, we cannot rule out the possible existence of urine contamination, measurement error, and other factors. After the sixth round of chemotherapy, serum FLC levels normalized, immunofixation electrophoresis was negative, and serum ALP levels declined to a nadir of 101 U/L. According to the response criteria, these outcomes constituted hematological stringent complete response (normal FLC ratio and negative serum and urine immunofixation, with FLC≤20 mg/L and dFLC≤10 mg/L) and complete organ response in the liver (ALP<2 times the lower limit of normal) [23], indicating the efficacy of low-frequency daratumumab. Remarkably, the time to first hematologic response and to complete response was short. Such a rapid response is associated with rapid elimination of toxic light chains leading to improved organ function [1,24]. Further, the time to hepatic response was significantly short. Previous studies have observed that in patients with AL amyloidosis with liver involvement, those with elevated total bilirubin levels have worse progressive-free survival and overall survival than do those with normal total bilirubin levels [6]. Total bilirubin levels normalized after daratumumab treatment in the present case, may indicate a better prognosis.
Conclusions
We present a case of AL amyloidosis with nonspecific symptoms. Liver involvement was confirmed on histological analysis of liver biopsy. The patient responded to a treatment regimen of low-frequency daratumumab and chemotherapy. The findings in the present case indicate that low-frequency daratumumab could be an effective and cost-effective treatment option for AL amyloidosis with liver involvement.
Figures
References:
1.. Merlini G, Dispenzieri A, Sanchorawala V, Systemic immunoglobulin light chain amyloidosis: Nat Rev Dis Primers, 2018; 4(1); 38
2.. He D, Guan F, Hu M, The clinical characteristics and prognosis of chinese patients with light-chain amyloidosis: A retrospective multicenter analysis: Indian J Hematol Blood Transfus, 2022; 38(3); 444-53
3.. Dispenzieri A, Merlini G, Immunoglobulin light chain systemic amyloidosis: Cancer Treat Res, 2016; 169; 273-318
4.. Kyle RA, Gertz MA, Primary systemic amyloidosis: Clinical and laboratory features in 474 cases: Semin Hematol, 1995; 32(1); 45-59
5.. Park MA, Mueller PS, Kyle RA, Primary (AL) hepatic amyloidosis: Clinical features and natural history in 98 patients: Medicine (Baltimore), 2003; 82(5); 291-98
6.. Zhang LL, Shen KN, Zhang CL, Clinical presentation and prognostic analysis of Chinese patients with systemic light chain amyloidosis with liver involvement: Leuk Res, 2019; 86; 106226
7.. de Weers M, Tai YT, van der Veer MS, Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors: J Immunol, 2011; 186(3); 1840-48
8.. Krejcik J, Casneuf T, Nijhof IS, Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma: Blood, 2016; 128(3); 384-94
9.. Kastritis E, Palladini G, Minnema MC, Daratumumab-based treatment for immunoglobulin light-chain amyloidosis: N Engl J Med, 2021; 385; 46-58
10.. Merlini G, Bellotti V, Molecular mechanisms of amyloidosis: N Engl J Med, 2003; 349(6); 583-96
11.. Gillmore JD, Wechalekar A, Bird J, Guidelines on the diagnosis and investigation of AL amyloidosis: Br J Haematol, 2015; 168(2); 207-18
12.. Sarsik B, Sen S, Kirdok FS, Hepatic amyloidosis: morphologic spectrum of histopathological changes in AA and nonAA amyloidosis: Pathol Res Pract, 2012; 208(12); 713-18
13.. Zhao L, Ren G, Guo J, The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement: Ann Med, 2022; 54(1); 1226-32
14.. Premkumar M, Rangegowda D, Vyas T, Primary hepatic amyloidosis presenting as acute-on-chronic liver failure: ACG Case Rep J, 2017; 4; e22
15.. Gertz MA, Kyle RA, Hepatic amyloidosis (primary [AL], immunoglobulin light chain): The natural history in 80 patients: Am J Med, 1988; 85(1); 73-80
16.. Rybinski B, Kocoglu M, Hepatic AL amyloidosis without significant light chain elevation in a patient treated with CyBorD plus daratumumab: Am J Case Rep, 2021; 22; e933241
17.. Ebert EC, Nagar M, Gastrointestinal manifestations of amyloidosis: Am J Gastroenterol, 2008; 103(3); 776-87
18.. Roussel M, Merlini G, Chevret S, A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis: Blood, 2020; 135(18); 1531-40
19.. Palladini G, Kastritis E, Maurer MS, Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: Safety run-in results of ANDROMEDA: Blood, 2020; 136(1); 71-80
20.. Sher T, Fenton B, Akhtar A, Gertz MA, First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis: Blood, 2016; 128(15); 1987-89
21.. Rajkumar SV, Dimopoulos MA, Palumbo A, International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma: Lancet Oncol, 2014; 15(12); e538-48
22.. Palladini G, Dispenzieri A, Gertz MA, New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes: J Clin Oncol, 2012; 30(36); 4541-49
23.. Muchtar E, Dispenzieri A, Leung N, Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria: Leukemia, 2018; 32(10); 2240-49
24.. Palladini G, Milani P, Malavasi F, Merlini G, Daratumumab in the treatment of light-chain (AL) amyloidosis: Cells, 2021; 10(3); 545
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250