19 March 2024: Articles 
Successful Anesthetic Management for Obese Patients with Interstitial Lung Disease Undergoing Laparoscopic Sleeve Gastrectomy: A Bridge to Improved Lung Transplant Eligibility
Unusual setting of medical care, Rare disease
Piotr Mieszczański1ABDEF*, Marek JaniakDOI: 10.12659/AJCR.942736
Am J Case Rep 2024; 25:e942736
Abstract
BACKGROUND: Patients with obesity with interstitial lung diseases (ILD) are encouraged to lose weight, as it improves lung function and lung transplant eligibility. As exercise tolerance in these patients is low and weight gain is a common adverse effect of corticosteroids, bariatric surgery can be an effective method for the management of obesity in this patient group. However, perioperative complications in such high-risk patients remain a concern. Therefore, we aimed to demonstrate successful anesthetic management for obese patients with ILD, which may be practically utilized to reduce perioperative pulmonary complications and improve outcomes.
CASE REPORT: Our case report presents a 42-year-old man with ILD who underwent laparoscopic sleeve gastrectomy (LSG). Preoperative studies revealed severe restrictive disease, right ventricular overload with assessed intermediate risk of pulmonary hypertension, and heart failure, with preserved left ventricle fraction but with poor exercise tolerance. Patient had opioid-free anesthesia (OFA) and postoperative multimodal analgesia. Following a 24-h stay in the Post-Anesthesia Care Unit, the patient was transferred to the ward and ultimately discharged home 2 days thereafter. At the 1-year follow-up, the patient reduced his weight by 40 kg and reported a significant improvement in physical capacity.
CONCLUSIONS: Our record demonstrates that OFA can be successfully used in high-risk patients with ILD undergoing LSG. In a period of a year, the patient improved so much that he no longer required lung transplantation, which may encourage clinicians to provide bariatric surgery using the OFA technique in the population of patients with obesity and severe respiratory illness.
Keywords: Obesity, Lung Transplantation, Bariatric Surgery, Anesthesia and Analgesia, idiopathic pulmonary fibrosis
Introduction
Interstitial lung diseases (ILD) are a broad spectrum of disorders characterized by scarring of the lung stromal tissue with restrictive ventilation defects, reduced diffusion capacity of the lungs, and impaired gas exchange [1]. Patients with ILD are characterized by a high prevalence of postoperative respiratory complications in both lung and non-lung surgeries [2]. In addition, obese patients undergoing bariatric surgery, including laparoscopic sleeve gastrectomy (LSG) are at particular risk of respiratory complications involving opioid-induced respiratory depression, atelectasis, and excessive sedation [3,4]. Taking into account the unique anesthetic challenges in this group of patients, the aim of this case report is to demonstrate their successful management using the opioid-free anesthesia (OFA) technique. Despite appropriate treatment, patients with ILD can progress to a life-threatening respiratory failure requiring a lung transplantation.
However, one of the factors that can significantly restrict eligibility and increase the risk of such management is obesity with a body mass index (BMI) of 35 or more [5], which can be associated with post-transplant increase in mortality and primary graft dysfunction [6]. Moreover, it has been proven that weight loss is one of the modifiable factors that improve the results of lung transplantation treatment and increase the chance of successful surgery [7,8]. As these risks diminish with the reduction of BMI values to 30 to 34.9 [7], patients are encouraged to lose weight to improve their pulmonary function tests and post-transplant survival [8,9]. Nevertheless, due to deteriorating exercise tolerance and adverse effects of prednisolone, the prevalence of obesity in patients with ILD is common [9]. For such patients, bariatric surgery can be an effective method of management of obesity and related metabolic derangements, with potential improvement of lung function and transplant eligibility [10].
Although there are reports in the literature on the role of bariatric surgery, including the most commonly performed LSG [11] as a bridging treatment to lung transplantation, the number of publications on this subject is limited [10,12,13] and they do not describe anesthetic management, which is crucial.
The administration of anesthesia and postoperative management poses several challenges, and thus, it is imperative to devise strategies to minimize risks. One such form of anesthetic technique, which can decrease the frequency of pulmonary complications and enhance patients’ recovery and safety, is OFA [14]. This article will present a case report of a patient with ILD on oxygen therapy who was successfully anesthetized for LSG as a bridge to lung transplant qualification using the opioid-free technique with multimodal postoperative analgesia.
Case Report
QUALIFICATION AND PREPARATION FOR SURGERY:
We report our experience concerning a 42-year-old male patient who had LSG. Written informed consent was obtained from the patient for the publication of this case report and accompanying images. The patient reported persistent cough, dyspnea, and deteriorating exercise toleration since 2007. The patient was a smoker, initially suspected of having chronic obstructive pulmonary disease, and ultimately received a diagnosis of respiratory bronchiolitis-associated ILD on the basis of the clinical image, high-resolution computed tomography (Figures 1, 2), pulmonary function tests, and lung biopsy. The differential diagnoses considered were non-specific interstitial pneumonia and desquamative interstitial pneumonia, with some of the histopathological features of these entities considerably overlapping. The cessation of smoking and the initiation of treatment with prednisolone and temporarily with azathioprine did not slow the progress of the disease, during which time the patient became oxygen-dependent.
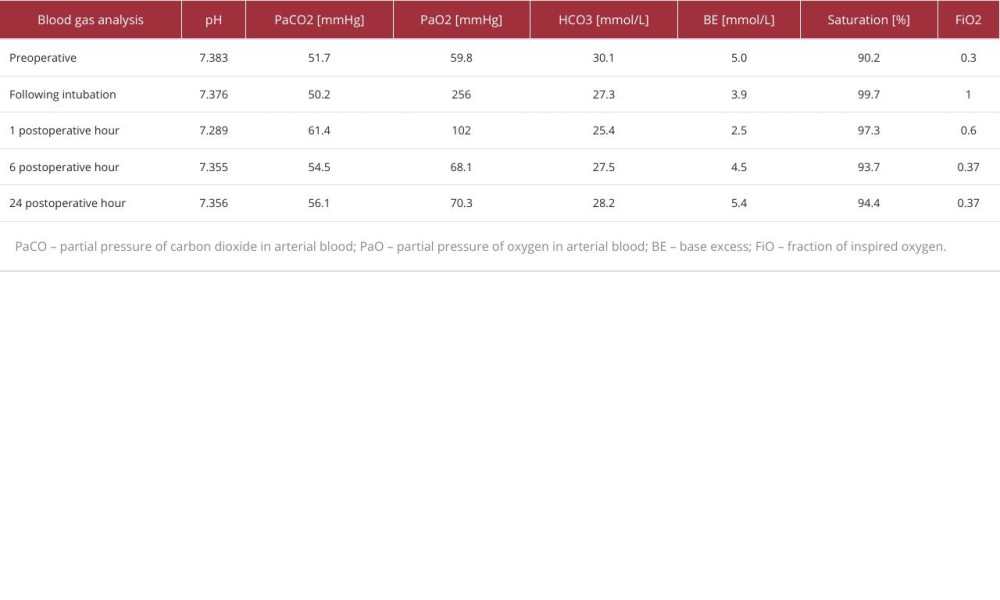
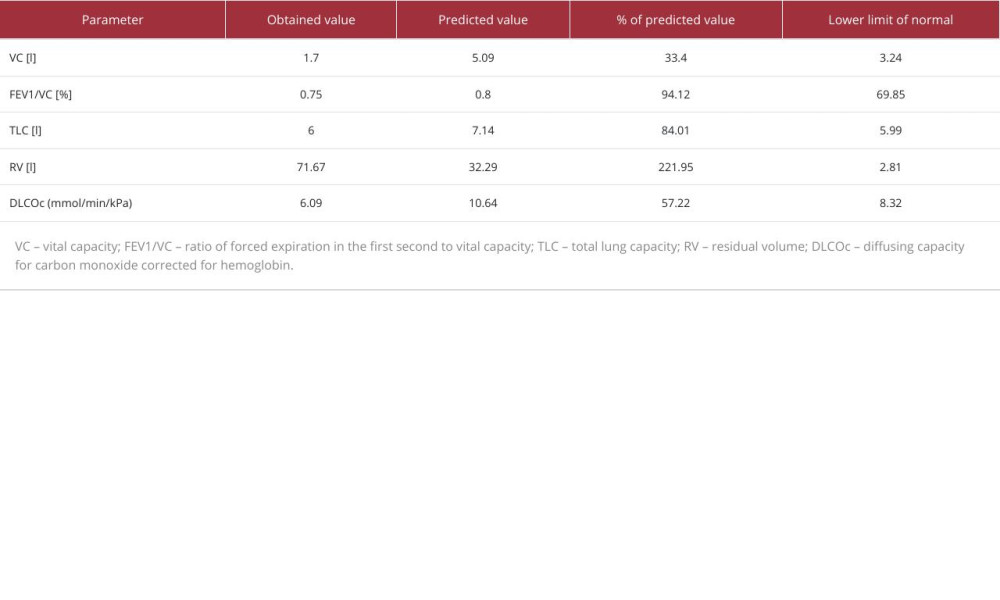
On initial qualification for LSG surgery, our patient had a BMI of 41.6 (height 178 cm, weight 132 kg) and required long-term oxygen therapy, receiving 5 L/min through nasal cannulas. Regarding concomitant diseases, the patient presented with hypertension, heart failure, with preserved ejection fraction and suspected pulmonary hypertension, diabetes treated with insulin, and non-alcoholic fatty liver disease. During preparation for surgery and anesthesia, the patient had arterial blood gas studies (Table 1), transthoracic echocardiography, and pulmonary function tests: spirometry, plethysmography, diffusion capacity assessment (Table 2), and a 6-min walk test.
Pulmonary function tests revealed a severe, restrictive ventilation defect with a moderate carbon monoxide transfer coefficient reduction. The 6-min walk test had to be stopped after 2 min 5 s due to intolerable dyspnea and leg cramps, with desaturation to 83% on oxygen therapy. In transthoracic echo-cardiography, right ventricular overload was present. Based on the measurement of the peak velocity of the tricuspid regurgitant wave, the risk of pulmonary hypertension was estimated as intermediate. Left ventricle contractility was preserved, with a left ventricle ejection fraction of 50% and hypokinesis of the lateral wall.
A multidisciplinary team consisting of a cardiologist, pulmonologist, and anesthetist stated that the patient was optimally prepared for bariatric surgery and was subsequently qualified for general anesthesia.
ANESTHETIC MANAGEMENT PREOPERATIVELY:
One hour before the surgery, the patient received pre-emptive analgesia with 1 g intravenous (i.v.) paracetamol and 2.5 g i.v. metamizole, adhering to Enhanced Recovery After Bariatric Surgery Society guidelines on LSG anesthesia [15]. We decided against giving dexamethasone, as the patient was already on prednisolone therapy for ILD.
In the operating room, before induction of the anesthesia, dexmedetomidine 70 mcg and 10-min infusion of lidocaine 100 mg i.v. was initiated. After positioning the patient in a benchmark position, preoxygenation with a high-flow nasal cannula (HFNC) was started, with FiO2 100% at a flow rate of 60 L/min. The anesthesia induction was performed by administering propofol 120 mg and ketamine 40 mg i.v. The ketamine was included as a component of multimodal analgesia due to its NMDA antagonist properties. A video laryngoscope was used, and the intubation was successful at the first attempt, maintaining saturation levels above 96% throughout the intubation procedure.
CONDUCTION OF ANESTHESIA:
Anesthesia was maintained using sevoflurane titrated to bispectral index values between 40 and 60. We decided against desflurane to avoid its irritating effect on the airways, and our team discussed using propofol total intravenous anesthesia, which was considered an alternative. Finally, we chose sevoflurane, owing to its potentially beneficial impact on lung mechanics, even though such an effect on patients undergoing LSG is debatable [16]. Following the induction of anesthesia, the radial artery was cannulated to perform arterial blood gas analyses and to monitor blood pressure invasively. Also, continuous infusion of dexmedetomidine 50 mcg and lidocaine 500 mg in 0.9% NaCl up to 50 mL in a single syringe was started, with infusion rates dependent on the hemodynamical parameters. We chose such a strategy to completely avoid opioids intraoperatively and potentially improve the patient’s recovery after the operation. During the surgery, magnesium sulfate 4 g i.v. was given, owing to its analgesic properties. To facilitate intubation and provide relaxation of muscles intraoperatively, rocuronium was dosed to keep a train-of-four ratio of 0. Mechanical ventilation was performed in the volume control ventilation mode, with an initial set tidal volume of 500 mL, frequency 16 breaths/ min, FiO2 50%, and positive end-expiratory pressure of 5 cmH2O.
At the beginning of the operation, the surgeons locally infiltrated the trocar insertion sites, with a total volume of 40 mL 0.25% bupivacaine. The surgical time was 65 min, and its course was uneventful. After placing the last sutures, sevoflurane was discontinued, and muscle relaxation was reversed with sugammadex to reach a train-of-four ratio of 4, with a 90% ratio between the first and fourth responses. We chose sugammadex over neostigmine to avoid muscarinic-related adverse effects and provide a more reliable and complete reversal of neuromuscular blockade. Upon regaining consciousness, the patient was extubated, and HFNC was reintroduced.
RECOVERY:
The infusion of dexmedetomidine and lidocaine was continued after the extubation and throughout the first 24 h of the Postoperative Care Unit (PACU) stay, with a reduced infusion rate of 4 mL/h (dexmedetomidine 4 mcg/h and lidocaine 40 mg/h i.v.). HFNC was continued, with FiO2 initially at 60%. On admission to PACU, the patient’s numeric pain score was 2/10.
After the first hour, the patient was over-sedated and somnolent. Arterial blood gas showed a rise of PaCO2 to 61.4 mmHg, probably due to loss of hypoxic respiratory drive (Table 1). We reduced FiO2 to 37% to reach SpO2 values of 88% to 90%, which enabled PaCO2 reduction and significantly improved the patient’s awareness.
To control the postoperative pain during the first 24 h in the PACU, simple analgesics were used, with fixed administration of metamizole 1g and paracetamol 1g every 6 h, and lidocaine and dexmedetomidine were given as an infusion, to cumulative doses of 1000 mg and 100 mcg, respectively. For any reported numeric pain score >3, oxycodone 2 mg i.v. boluses were given. During his stay in the PACU, the patient required 6 mg of oxycodone in total, whereas the maximal numeric pain score was 6/10.
Following a 24-h stay in the PACU, the patient was subsequently transferred to the ward and ultimately discharged home 2 days thereafter.
In the follow-up visit 1 month after the surgery, a loss of 19 kg in body weight was noted, and the patient was referred to start the procedure of the qualification for lung transplantation. At the 1-year follow-up, the patient reduced his body weight by 40 kg in total, required oxygen therapy only at night, with a decreased flow of 1 L/min, and reported a significant improvement in physical capacity to a level that he could return to professional work. In response to this progress, a multidisciplinary decision was made to suspend the lung transplantation qualification.
Discussion
The presented case study provides evidence of effective anesthetic management, utilizing the combination of OFA and postoperative multimodal analgesia, in a patient with grade III obesity, ILD, and comorbidities who had a significantly increased risk for respiratory complications. To the best of our knowledge, the anesthetic technique of such a patient has not been previously published in the literature.
In planning general anesthesia for the described patient, our primary objective was to mitigate the potential for complications arising from anesthesia and the risk of severe hypoxia during the induction and postoperative periods. One of the modifiable factors that significantly affects postoperative respiratory complications is opioid use. Clinically relevant disorders of respiratory mechanics can occur following opioid use, even in the absence of overdose symptoms [17]. To address this issue, the Enhanced Recovery After Bariatric Surgery Society guidelines recommend multimodal analgesia, the administration of co-analgesics, regional anesthesia, and non-opioid analgesics [4,18]. These agents, in combination, make it possible to eliminate the intraoperative use of opioids, which is referred to as OFA [4].
The co-analgesics we used were the alpha-2 agonist dexmedetomidine, lidocaine, and NMDA antagonists ketamine and magnesium sulfate. As these drugs have an independent analgesic effect or potentiate the effect of other analgesics, their administration, especially after surgery, can optimize pain management while reducing opioid use and mitigating the risk of their adverse effects, such as respiratory failure [14,19], postoperative nausea and vomiting [14,20–22], or opioid-induced hyperalgesia [23]. Therefore, this allows for faster convalescence after surgery and anesthesia and early mobilization, an essential element of the Enhanced Recovery After Surgery protocol [14,18]. Nevertheless, the impact of OFA on recovery remains controversial, as in a recent meta-analysis dedicated to bariatric surgery, the improvement in this field was debatable [24]. The paper, however, had significant limitations due to high heterogeneity and did not encompass the most recent study, in which co-analgesics administration was prolonged in the PACU, with a significant improvement in the quality of recovery score [25]. We consider the continuation of co-analgesics infusion as a pivotal element affecting the potential benefits in safety and recovery, as in some studies with repeated pain score assessment, the opioid-reducing effect was diminished immediately after cessation of the co-analgesic infusion [14,20].
When administering co-analgesics in OFA, it is crucial to consider their potential adverse effects, especially their impact on the circulatory system. These medications can induce hypo-tension [26–28] and escalate the need for vasopressors [20]. Therefore, it is imperative to closely monitor patients receiving these medications and manage any adverse effects promptly. In our case, the co-analgesics that were used may have compromised the hemodynamic condition of the patient that had chronic heart failure and an increased risk of pulmonary hypertension, as assessed by echocardiography [29,30]. However, after discussion, we decided that the potential benefits outweighed the risks, and administering the abovementioned drugs was carried out under continuous invasive blood pressure measurement, with mean arterial pressure values maintained above 65 mmHg during all stages of anesthesia.
In the presented case, we used a relatively low dose of dexmedetomidine, considering the dose-dependent risk of hypotension, bradycardia, excessive sedation, and increased desaturation rates [26]. In the study by Beloil et al, the dexmedetomidine dose was much higher with prolonged administration, which could have attributed to the reported adverse effects [31]. Moreover, in our presented case, we decided to discontinue the administration of ketamine after induction of anesthesia, as even small doses of this drug can trigger hallucinations [21].
An important factor in improving the patient’s safety was the use of HFNC in the pre-oxygenation and postoperative periods. Our case aligns with the evidence that HFNC prolongs the safe apnea time during induction [32], and we demonstrated its practical use. After surgery, HFNC at a flow rate of 60 L/min allowed for a potential for atelectasis reduction and better CO2 flushing. Nevertheless, we were not able to avoid transient hypercapnia caused by excessive PaO2, but this was successfully managed by lowering FiO2, as demonstrated in the arterial blood gas at 6 h and 24 h after surgery (Table 1).
Although there was a transient aggravation of respiratory failure in the first hour after surgery, our case outcome was favorable. Afraz et al demonstrated that patients on oxygen therapy had significantly higher mortality and morbidity after bariatric surgery [33]; our report could contribute to the effective management of this increased risk during the perioperative period. The presented approach may also prove valuable in planning the anesthesia of such oxygen-dependent bariatric patients in other centers, as well as encouraging qualifying them for bariatric surgery.
A limitation of this study is that it is a single case report, which, by its nature, restricts the generalizability of our clinical approach. Given the very small number of studies on safe anesthetic techniques such as OFA in obese patients with ILD undergoing bariatric surgery, more high-quality observational and randomized controlled studies are warranted for an evidence-based approach in this patient population.
Conclusions
This case report demonstrates a possible successful anesthesia management in an unusual clinical scenario in a high-risk patient with ILD undergoing LSG as a bridge to lung transplantation. Our record reveals that, due to OFA with multi-modal postoperative analgesia and HFNC perioperatively, safe performance of bariatric procedure was achievable, which resulted in immense improvement in the patient’s condition, including a return to professional activity and avoidance of lung transplantation. We hope that this case report encourages clinicians to provide bariatric surgery in the population of patients with obesity and concomitant severe respiratory illness.
References:
1.. Wallis A, Spinks K, The diagnosis and management of interstitial lung diseases: Br Med J, 2015; 350; h2072
2.. Choi SM, Lee J, Park YS, Postoperative pulmonary complications after surgery in patients with interstitial lung disease: Respiration, 2014; 87(4); 287-93
3.. Gupta K, Nagappa M, Prasad A, Risk factors for opioid-induced respiratory depression in surgical patients: A systematic review and meta-analyses: BMJ Open, 2018; 8(12); e024086
4.. Mulier J, Dekock M, Opioid free general anesthesia, a new paradigm?: Best Pract Res Clin Anaesthesiol, 2017; 31(4); 441-43
5.. Leard LE, Holm AM, Valapour M, Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation: J Heart Lung Transplant, 2021; 40(11); 1349-79
6.. Upala S, Panichsillapakit T, Wijarnpreecha K, Underweight and obesity increase the risk of mortality after lung transplantation: A systematic review and meta-analysis: Transpl Int, 2016; 29(3); 285-96
7.. Chandrashekaran S, Keller CA, Kremers WK, Weight loss prior to lung transplantation is associated with improved survival: J Heart Lung Transplant, 2015; 34(5); 651-57
8.. Clausen ES, Frankel C, Palmer SM, Pre-transplant weight loss and clinical outcomes after lung transplantation: J Heart Lung Transplant, 2018; 37(12); 1443-47
9.. Sekine A, Wasamoto S, Hagiwara E, Beneficial impact of weight loss on respiratory function in interstitial lung disease patients with obesity: Respir Investig, 2021; 59(2); 247-51
10.. Ardila-Gatas J, Sharma G, Nor Hanipah Z, Bariatric surgery in patients with interstitial lung disease: Surg Endosc, 2019; 33(6); 1952-58
11.. Brown AW, Shikora S, Liem R, International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). 7th IFSO Global Registry Report [monograph on the Internet], 2022 Available from: https://www.ifso.com/pdf/ifso-7th-registry-report-2022.pdf
12.. Takata MC, Campos GM, Ciovica R, Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation: Surg Obes Relat Dis, 2008; 4(2); 159-65
13.. Martin MJ, Bennett S, Pretransplant bariatric surgery: A new indication?: Surg Obes Relat Dis, 2007; 3(6); 648-51
14.. Mulier JP, Wouters R, Dillemans B, Dekock M, A randomized controlled, double-blind trial evaluating the effect of opioid-free versus opioid general anaesthesia on postoperative pain and discomfort measured by the QoR-40: J Clin Anesth Pain Med, 2018; 6; 2
15.. Macfater H, Xia W, Srinivasa S, Evidence-based management of postoperative pain in adults undergoing laparoscopic sleeve gastrectomy: World J Surg, 2019; 43(6); 1571-80
16.. Öztürk MC, Demiroluk Ö, Abitagaoglu S, Ari DE, The Effect of sevoflurane, desflurane and propofol on respiratory mechanics and integrated pulmonary index scores in laparoscopic sleeve gastrectomy. A randomized trial: Saudi Med J, 2019; 40(12); 1235-41
17.. Overdyk F, Dahan A, Roozekrans M, Opioid-induced respiratory depression in the acute care setting: A compendium of case reports: Pain Manag, 2014; 4(4); 317-25
18.. Stenberg E, Dos Reis Falcão LF, O‘Kane M, A 2021 Update [published correction appears in World J Surg. 2022;46(4):752]: World J Surg, 2022; 46(4); 729-51
19.. Bhardwaj S, Garg K, Devgan S, Comparison of opioid-based and opioid-free TIVA for laparoscopic urological procedures in obese patients: J Anaesthesiol Clin Pharmacol, 2019; 35(4); 481-86
20.. Mieszczański P, Górniewski G, Ziemiański P, Comparison between multimodal and intraoperative opioid free anesthesia for laparoscopic sleeve gastrectomy: A prospective, randomized study: Sci Rep, 2023; 13(1); 12677
21.. Mansour MA, Mahmoud AA, Geddawy M, Nonopioid versus opioid based general anesthesia technique for bariatric surgery: A randomized double-blind study: Saudi J Anaesth, 2013; 7(4); 387-91
22.. Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT, Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis: Br J Anaesth, 2014; 112(5); 906-11
23.. Fletcher D, Martinez V, Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis: Br J Anaesth, 2014; 112(6); 991-1004
24.. Hung KC, Chiu CC, Hsu CW, Impact of opioid-free anesthesia on anal-gesia and recovery following bariatric surgery: A meta-analysis of randomized controlled studies: Obes Surg, 2022; 32(9); 3113-24
25.. Ulbing S, Infanger L, Fleischmann E, The performance of opioid-free anesthesia for bariatric surgery in clinical practice: Obes Surg, 2023; 33(6); 1687-93
26.. Beloeil H, Garot M, Lebuffe G, Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery: Anesthesiology, 2021; 134(4); 541-51
27.. Omar AM, Can systemic lidocaine be used in controlled hypotension? A double-blinded randomized controlled study in patients undergoing functional endoscopic sinus surgery: Egypt J Anaesth, 2013; 29; 295-300
28.. Elsharnouby NM, Elsharnouby MM, Magnesium sulphate as a technique of hypotensive anaesthesia: Br J Anaesth, 2006; 96(6); 727-31
29.. Price LC, Martinez G, Brame A, Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: A systematic review and expert consensus statement: Br J Anaesth, 2021; 126(4); 774-90
30.. Walsh M, Devereaux PJ, Garg AX, Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension: Anesthesiology, 2013; 119(3); 507-15
31.. Forget P, Mulier J, Lavand’homme P, Opioid-free anesthesia: Comment: Anesthesiology, 2021; 135(4); 751-53
32.. Wong DT, Dallaire A, Singh KP, High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: A randomized controlled trial: Anesth Analg, 2019; 129(4); 1130-36
33.. Afraz S, Dang JT, Modasi A, Bariatric surgery outcomes in oxygen-dependent patients: Analysis of the MBSAQIP database: Surg Obes Relat Dis, 2019; 15(9); 1571-80
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250