23 April 2024: Articles 
Eosinophilic Pleural Effusion Secondary to Infection in a Patient with Systemic Sclerosis: A Case Report
Challenging differential diagnosis, Unusual setting of medical care, Rare coexistence of disease or pathology
Mădălina Ștefania VulcanDOI: 10.12659/AJCR.943420
Am J Case Rep 2024; 25:e943420
Abstract
BACKGROUND: Scleroderma is a chronic autoimmune disease characterized by angiopathy, autoimmunity, and fibrosis. One form of scleroderma, systemic sclerosis, is characterized by diffuse skin lesions and visceral involvement. Eosinophilic pleural effusion is a rare complication attributed to a large array of diseases. We present a case of a man with underlying systemic sclerosis who developed eosinophilic pleural effusion as a complication of associated Trichinella spiralis infection.
CASE REPORT: A 49-year-old man presented for bilateral inflammatory radio-ulnar-carpal joint pain, paresthesia of the hands and forearms and a 2-week history of right posterior aching thoracic pain and night sweats. The physical examination revealed sclerodermatous skin involvement of the hands, forearms, and forehead, sclerodactyly, Raynaud’s phenomenon, and telangiectasias, together with muffled cardiac sounds and right basal abolishment of the vesicular breath sounds. Imagistic evaluation showed the presence of pleuro-pericardial fluid. A thoracocentesis highlighted the presence of an exudative eosinophilic pleural effusion. Laboratory findings showed leukocytosis, with elevated neutrophil and eosinophil counts. The patient was tested for a parasitic infection, but initially the results were negative. He started anti-inflammatory treatment, but no reduction of the pleural fluid was observed. Subsequent evaluation revealed specific anti-trichinella IgG antibodies. Albendazole and corticosteroid therapy were initiated, which resulted in remission of the symptoms.
CONCLUSIONS: This report highlights the possibility of developing rare or even not-until-now seen complications when 2 etiologically different diseases are associated. The physician should carefully assess the situation to find and resolve the underlying causes.
Keywords: Eosinophilia, Pleural Effusion, Scleroderma, Systemic, Trichinella spiralis
Introduction
Scleroderma is a chronic disease characterized by 3 pathological mechanisms: small-vessels angiopathy, autoimmunity, and excessive fibrosis. It can be classified into 2 forms based on the extension of the disease: localized scleroderma and systemic scleroderma (systemic sclerosis, SSc) [1,2].
SSc is clinically characterized by skin lesions (skin thickening of the fingers, digital ulcerations, telangiectasias), Raynaud’s phenomenon, and respiratory dysfunction (eg, interstitial lung disease, pulmonary arterial hypertension). Elevated levels of anti-centromere and anti-topoisomerase I antibodies are related to the presence of the disease. Disorders such as gastroesophageal reflux disease, myocardial disease, arrhythmias, pericardial disease, and renal involvement are complications of SSc [1,2]. Pleural involvement (eg: pleural effusion, diffuse pleural thickening, subpleural micronodular lesions) is not specific to SSc but can occur and is usually associated with pulmonary arterial hypertension (PAH). While pleural effusion and pleural thickening are general findings in SSc, they are not common findings on chest radiography, and significant pleural effusion is considered to be uncommon [3].
Eosinophilic pleural effusion (EPE) is defined by an eosinophil count of at least 10% in the pleural fluid cytology analysis [4]. EPE is a rare manifestation with a broad spectrum of etiologies, such as malignancy (metastatic carcinoma, malignant mesothelioma, malignant lymphoma, malignant myeloma), blood/air in the pleural space, infectious diseases (
In this report we present a case of systemic sclerosis complicated with
Case Report
A 49-year-old man with a past medical history of arterial hyper-tension presented for bilateral inflammatory radio-ulnar-carpal joint pain, paresthesia of the hands and forearms, and fatigue. He also had a 6-month history of Raynaud’s phenomenon and a 2-week history of right posterior aching thoracic pain and night sweats. Additionally, he did not present a history of parasitic infections or of travel outside of Europe. The patient was stable, with heart rate of 98 beats/minute, blood pressure of 114/70 mmHg, and oxygen saturation of 94%. The initial physical exam was notable for substantial sclerodermatous skin involvement of the fingers (mRSS=3), hands (mRSS=2), forearms (mRSS=1), and forehead (mRSS=2), with a total of 14 points of mRSS score, sclerodactyly, Raynaud’s phenomenon, telangiectasias (Figure 1), metacarpophalangeal (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) swelling. A closer evaluation revealed diffuse reduction of the vesicular breath sounds, bilateral basal dullness to percussion and basal abolishment of the vesicular breath sounds, and edema of the hands and legs. He was a former smoker (35 pack-years) and a chronic alcohol consumer, and he was exposed to toxic substances due to work (construction).
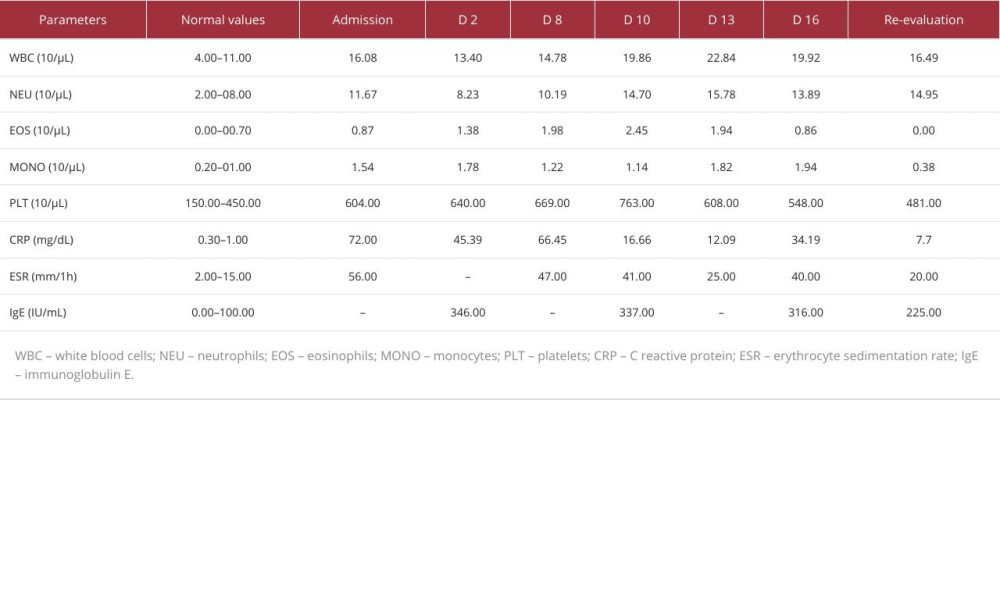
Table 1 shows the laboratory findings at admission. Antinuclear antibodies (ANAs) and anti-topoisomerase I antibodies (anti-Scl 70) levels were significantly elevated (ANA=554.27 IU/mL, anti-Scl 70=179.19 IU/mL). Other tests, including blood urea nitrogen (BUN), uric acid, creatinine, serum complement proteins (C3, C4), creatine-kinase (CK), lactate dehydrogenase (LDH), thyroid-stimulating hormone (TSH), free thyroxine (fT4), and the electrolyte panel were normal. At admission, the ECG showed sinus rhythm with micro-voltage QRS complexes. A chest X-ray revealed accentuation of interstitial lung tissue and left diaphragmatic flattening and left costophrenic recess obstruction, spirometry and diffusing capacity of the lungs for carbon monoxide were within normal parameters (forced vital capacity 109%, forced expiratory volume in 1 s 98%, diffusing capacity of the lungs for carbon monoxide [DLCO] 91%, total lung capacity during DLCO 90%) and a thorax high-resolution CT (HRCT-scan) showed bilateral pleurisy, pericardial effusion, and minimal nonspecific interstitial lesions (Figures 2, 3). The nonspecific interstitial lung impairment was analyzed as pulmonary involvement, possibly due to toxic exposure (materials and substances used in construction, nicotine) and re-evaluation of the pulmonary system was scheduled as an integral part of the monitoring plan. Under those circumstances, based on the clinical presentation and the imaging findings, the preliminary diagnosis was scleroderma.
An ultrasound examination was conducted and confirmed the presence of pleural and pericardial effusion. The following day, an ultrasound re-examination revealed fluid volume expansion in the right pleural cavity and a diagnostic thoracentesis was performed. The pleural fluid analysis was negative for a bacterial infection but the presence of an exudate with high concentration of eosinophils on the fluid cytology analysis was observed (LDH=1387.00 IU/mL, C3=0.51 g/L, C4=0.05 g/L, 10% eosinophils). Blood tests were repeated and showed an elevated level of IgE (346.00 IU/mL), together with a decrease of the white blood cells level (13.40×103/μL) and an increase of the eosinophils count (1.38×103/μL). We tried to integrate hypereosinophilia etiologically. The patient underwent specialty parasitological consultation and had a stool ova and parasite (O&P) examination, but the results were negative, with no ova, larvae, or parasite cysts being identified. Additionally, no familial epidemiologic context was found. A hematology consultation was recommended, and a bone marrow biopsy examination was done to rule out a myelodysplastic syndrome (MDS). The bone marrow was cellular-rich, had polymorphic appearance, and was characterized by a hyperplastic granulocytic series with present maturation and mild eosinophilia, with no signs of atypical cytology. We considered testing for FIP1L1-PDGFRA fusion gene, but once the eosinophilia improved under corticosteroid and anthelmintic therapy, we ruled out hypereosinophilic syndrome as an etiology. Multiple ultrasound examinations were done and each showed slightly progressive bilateral pleural fluid volume expansion.
Although the clinical presentation and evolution of the patient were not specific, we again searched for a cause of the parasitosis. As a result, we re-evaluated the patient, with a major focus on targeting his activities and recent history, and we discovered a history of raw meat consumption in January, 7 months prior to his admission. We tested him for specific anti-trichinella IgG antibodies and the results were positive; therefore, after another parasitological consultation, it was decided that the history of raw meat consumption, eosinophilia, and positive serology (IgG antibodies) were enough for a diagnosis of
Initially, we attempted addressing the symptomatology; therefore, nonsteroidal inflammatory drugs and vasodilator agents, such as pentoxifylline 400 mg per day, ketoprofen 100 mg/2 ml per day, and acetaminophen 1000 mg/100 mL per day were administered. After
Discussion
Systemic sclerosis is a rare autoimmune condition with a broad range of age onset (30–50 years) [2]. It is most common in the indigenous population in Canada (47/100.000), followed by Europeans, and affects more females than males (4.6: 1) [9]. Men are prone to have diffuse form, pulmonary, cardiac and renal impairment, while women are more likely to have the limited form [2]. This connective tissue disorder remains, nonetheless, a rare disease well known for its heterogeneity regarding clinical manifestations, organ involvement, progression, severity, and outcomes [10]. The diagnosis and classification of SSc is based on the ACR-EULAR score. Our patient presented bilateral skin thickening of the fingers that extended proximally to the MCP joints, which is sufficient to make the diagnosis of SSc [1]. Additionally, he had fingertip pitting scars, telangiectasia, Raynaud’s phenomenon, anti-topoisomerase I antibodies, and a potential interstitial lung disease (based on the chest X-ray), with a total score of 22, more than enough to make the diagnosis of systemic sclerosis [1]. The disease is characterized by vasculopathy, produced by antibody-mediated endothelial apoptosis and endothelial dysfunction, the latter causing an imbalance between vasoconstrictor and vasodilator agents [11,12]. This triggers Raynaud’s phenomenon early in the disease progression, before the onset of sclerosis, as seen in our case [11]. Cardiac complications can be present as a primary heart impairment, but most frequently they are encountered in association with pulmonary hypertension, being clinically silent in over 70% cases [13]. Pericarditis is common (up to 70%), can be acute or chronic, and often arises in the form of pericardial effusion [14]. Our patient showed no signs of increased blood pressure in the arteries of the lung and no symptoms of pericardial inflammation, but the abnormalities of cardiac auscultation, ECG, and echocardiography proved the moderate accumulation of pericardial fluid.
Eosinophilic pleural effusions (EPE) represent 5% to 16% of exudative pleural effusions [4]. In a 2012 meta-analysis, the most common causes of EPE were malignancy (26%), idiopathic (25%), and parapneumonic (13%) effusions, followed by pleural air/blood (13%), tuberculosis (7%), collagen vascular diseases (3%), as well as eosinophilic lung disease or infections [6].
A recent retrospective study observed a higher prevalence of malignancy (52.94%) and of pleural parasitic infestations (8.82%) [7]. In our patient, no malignant formation could be seen on the thorax CT scan, and no other imaging evaluation showed the presence of a tumoral process. The patient’s history, clinical presentation, and laboratory findings were not consistent with pneumonia, tuberculosis, or systemic autoimmune diseases associated with EPE. Although an autoimmune disease, scleroderma has not been reported to cause EPE to the best of our knowledge. As such, we searched for the presence of a parasitic infection. Among the parasitic diseases, the most associated with EPE is paragonimiasis; other diseases include cutaneous myiasis, echinococcosis, strongyloidiasis, amebiasis, toxocariasis, and filariasis [4,15]. Initially, the patient had a stool O&P examination, which tested the presence of
While SSc and trichinosis are 2 etiologically different diseases, they show similarities regarding their immune system activation pathway, the most evident being the amplified response of type 2 T helper cells (Th2). This translates to an elevated level of cytokines, especially of interleukins 4 (IL-4) and inter-leukin 13 (IL-13), which are observed in high titers in both conditions [17–19]. On the one hand, both these cytokines are fibrogenic factors in SSc, by stimulating the B cells production of immunoglobulins and activating the fibroblast proliferation and differentiation pathways, thus enhancing the fibrosis [19]. Additionally, they have been observed to enhance a series of inflammation-related mechanisms [19]. On the other hand, in trichinellosis, immune cells populations, such as T cells, fibro-blasts, eosinophils, basophils, and macrophages, are recruited by specific antibody subclasses and the elevated IL-4 and IL-13, which in turn determine the development of hypersensitivity reactions. Our hypothesis was that by having these similarities in the immune system activation, the concomitant presence of the 2 diseases determined an amplified inflammatory response. It is not certain if the specific combination of the 2 diseases led to the heterogeneity of clinical manifestations or if one of them is associated with the rarer clinical presentations, such as EPE. To the best of our knowledge, there are no reported cases of SSc or
Conclusions
We presented a case of a man with systemic sclerosis and
Figures
References:
1.. Van Den Hoogen F, Khanna D, Fransen J, 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League Against Rheumatism collaborative initiative: Arthritis Rheum, 2013; 65; 2737-47
2.. Odonwodo A, Badri T, Hariz A, Scleroderma. [Updated 2023 Jul 31]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK537335/
3.. Farrokh D, Abbasi B, Fallah-Rastegar Y, Mirfeizi Z, The extrapulmonary manifestations of systemic sclerosis on chest high resolution computed tomography: Tanaffos, 2015; 14; 193-200
4.. Kalomenidis I, Light RW, Eosinophilic pleural effusions: Curr Opin Pulm Med, 2003; 9(4); 254-60
5.. de Ghellinck L, Frusch N, Duysinx B, Eosinophilic pleural effusion induced by paliperidone palmitate: Case report and literature review: J Acad Consult Liaison Psychiatry, 2022; 63(4); 394-99
6.. Oba Y, Abu-Salah T, The prevalence and diagnostic significance of eosinophilic pleural effusions: A meta-analysis and systematic review: Respiration, 2012; 83(3); 198-208
7.. Li M, Zeng Y, Li Y, Incidence, aetiology and clinical features of eosinophilic pleural effusion: A retrospective study: BMC Pulm Med, 2021; 21(1); 402
8.. Butt NM, Lambert J, Ali S, Guideline for the investigation and management of eosinophilia: Br J Haematol, 2017; 176(4); 553-72
9.. Calderon LM, Pope JE, Scleroderma epidemiology update: Curr Opin Rheumatol, 2021; 33(2); 122-27
10.. Varga J, Clinical manifestations and diagnosis of systemic sclerosis (scleroderma) in adults: UpToDate, Wolters Kluwer (Accessed Jan 2024)
11.. Furue M, Mitoma C, Mitoma H, Pathogenesis of systemic sclerosis – current concept and emerging treatments: Immunol Res, 2017; 65(4); 790-97
12.. Christopher P, Denton M, Pathogenesis of systemic sclerosis (scleroderma): UpToDate, Wolters Kluwer (Accessed Jan 2024).
13.. Massimo Imazio MFF, Pericardial involvement in systemic autoimmune diseases: UpToDate, Wolters Kluwer (Accessed Jan 2024)
14.. Yoshimi R, Nakajima H, The treatment of systemic sclerosis-related pericarditis: Intern Med, 2022; 61; 2997-98
15.. Pan ZZ, Zhu MJ, Rong YQ, Yang J: BMC Infect Dis, 2023; 23(1); 77
16.. Bruschi F, Gómez-Morales MA, Hill DE, International Commission on Trichinellosis: Recommendations on the use of serological tests for the detection of Trichinella infection in animals and humans: Food Waterborne Parasitol, 2019; 14; e00032
17.. Bruschi F, Ashour DS, Othman AA, Trichinella-induced immunomodulation: Another tale of helminth success: Food Waterborne Parasitol, 2022; 27; e00164
18.. Sofronic-Milosavljevic L, Ilic N, Pinelli E, Gruden-Movsesijan A: J Immunol Res, 2015; 2015; 523875
19.. Rosendahl A-H, Schönborn K, Krieg T, Pathophysiology of systemic sclerosis (scleroderma): Kaohsiung J Med Sci, 2022; 38(3); 187-95
Figures
In Press
28 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942881
28 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943590
29 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943843
30 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943577
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250