13 March 2024: Articles 
A 65-Year-Old Man with Refractory Hemoptysis Associated with Chronic Progressive Pulmonary Aspergillosis Who Failed to Respond to Combined Endobronchial Occlusion and Bronchial Artery Embolization: A Case Report and Literature Review
Management of emergency care
Ryotaro Yoneoka1ABCDEF, Kenichiro Takeda2ABCDEF, Hajime KasaiDOI: 10.12659/AJCR.942422
Am J Case Rep 2024; 25:e942422
Abstract
BACKGROUND: Hemoptysis due to airway hemorrhage is treated with hemostatic agents, bronchial artery embolization (BAE), or surgical resection. We present the case of a 65-year-old man with refractory hemoptysis associated with chronic progressive pulmonary aspergillosis (CPPA) who failed to respond to combined endobronchial occlusion (EBO) with endobronchial Watanabe spigot (EWS) and BAE.
CASE REPORT: A 63-year-old man was diagnosed with CPPA in the right upper lung and presented to our hospital 2 years later for hemoptysis at age 65. He developed severe hemoptysis during an outpatient visit, and was urgently admitted, intubated, and ventilated to prevent choking on blood clots. Chest computed tomography showed a large mass in the apical portion of the right lung, constituting apical pleural thickening and an encapsulated pleural effusion, and dilatation in the bronchial artery supplying the right upper lung lobe. Bronchoscopy revealed the right upper lobe B1-B3 as the bleeding source. The patient had recurrent hemoptysis that was not controlled by BAE or 6 EBO+EWS procedures, and he ultimately died of hypoxemia. In the literature review, EBO+EWS can effectively control hemoptysis in appropriate cases, without the need for BAE or surgical lung resection. It is less invasive, is associated with fewer adverse events than BAE or surgery, and can achieve temporary hemostasis for severe hemoptysis.
CONCLUSIONS: BAE and EBO+EWS were ineffective in controlling recurrent hemoptysis caused by CPPA in this case. However, a multidisciplinary approach such as attempting hemostasis with combined EBO+EWS and BAE may be a viable treatment option in severe cases of hemoptysis.
Keywords: Bronchoscopy, Hemoptysis, pulmonary aspergillosis
Background
Hemoptysis is a symptom of airway hemorrhage and can be fatal [1]. It is generally treated with hemostatic agents, bronchial artery embolization (BAE), surgical resection, or a combination of these procedures [2]. However, BAE may not provide adequate hemostasis, and treatable vessels may not be present in some cases [3]. Moreover, surgical resection is not advisable for patients with poor general health or reduced lung function. Endobronchial occlusion (EBO) involves physically occluding the bronchus responsible for the hemoptysis with an embolizing substance and applying pressure hemostasis [2]. EBO can be performed with bronchial blockers [4]. The endobronchial Watanabe spigot (EWS) is a silicone filter for refractory pneumothorax and bronchial fistulas and can be used to manage hemoptysis [5,6]. To perform EBO with an EWS, a bronchoscope is used to fill the bleeding bronchus with an EWS, which is grasped by forceps while the patient is intubated [6]. Reports on cases of hemoptysis managed by EBO with EWS (EBO+EWS) are limited [5,7–27]. Two types of cases exist in which BAE and EBO are combined to achieve hemostasis in hemoptysis. First, EBO is performed to symptomatically stop the bleeding, and BAE is subsequently performed for curative purposes [15–17,17,23,24,27]. Second, BAE is first performed as a radical therapy to achieve hemostasis; however, hemoptysis is not always controlled with this method, and EBO may be performed as an adjunctive additional treatment. Furthermore, BAE may be added after EBO achieves temporary hemostasis [7,9,13,19,20,22,26].
In this report, we presented a case of a 65-year-old man with refractory hemoptysis associated with chronic progressive pulmonary aspergillosis (CPPA) who failed to respond to combined EBO+EWS and BAE. Furthermore, we discussed the effectiveness of EBO+EWS based on a literature review of cases in which EBO+EWS was performed for hemoptysis.
Case Report
A 63-year-old man presented to our hospital for hemoptysis. He was diagnosed with CPPA based on imaging findings of progressively worsening infiltrative shadows over 4 years that were unresponsive to antimicrobial agents, and positive serum anti-aspergillus antibodies. CPPA was treated with voriconazole, which was discontinued after 7 months owing to liver dysfunction. Thereafter, the patient’s CPPA gradually worsened, and he presented to our hospital for hemoptysis at age 65 years. He had been previously diagnosed with polyneuropathy-organomegaly-endocrinopathy-monoclonal gammopathy-skin abnormalities syndrome 16 years ago and had undergone treatment for 12 years, which included high-dose dexamethasone, hematopoietic stem cell transplantation, and lenalidomide. Subsequently, his blood vascular endothelial growth factor level remained in the normal range. Additionally, he had type 2 diabetes mellitus and a history of myocardial infarction. He had been taking oral aspirin and clopidogrel until the time of his massive hemoptysis.
The patient developed severe hemoptysis during his outpatient visit and was urgently admitted to the hospital. He was intubated and ventilated to prevent choking on blood clots and was admitted to the high care unit (HCU). Chest radiography showed infiltration in the right upper lung field (Figure 1A). Chest computed tomography showed a large mass in the apical portion of the right lung, constituting apical pleural thickening, and an encapsulated pleural effusion in the mass. Additionally, the mass in the apex of the right lung had enlarged over time. In addition, the bronchial artery supplying the right upper lobe of the lung was dilated (Figure 1B). Bronchoscopy revealed that the source of bleeding was the right upper lobe B1-B3. He was treated with tranexamic acid, and antiplatelet agents were discontinued. Antimicrobial agents, including antifungals, ampicillin/sulbactam, cefepime, clindamycin, and voriconazole, were administered. BAE was performed first due to the severity of the hemoptysis, which resulted in temporary control, and digitally subtracted angiography demonstrated that the right bronchial arteries were abnormal, with hypertrophy and peripheral bronchopulmonary shunt. Embolization of the right bronchial artery was performed with use of porous gelatin particles (Gelpart, Nippon Kayaku, Tokyo, Japan) (Figure 2). However, the hemoptysis recurred. The patient was considered for pulmonary resection; however, surgery was not performed owing to his poor lung function and general condition from CPPA progression. Additionally, surgery would have been technically difficult since multiple new vessels had grown from the chest wall. Therefore, EBO+EWS was performed in the HCU on day 6 of hospitalization using an aScope 4 Broncho Regular 5.0/2.2 (Ambu® A/S, Ballerup, Denmark) while the patient was intubated. Sedation with midazolam and fentanyl was performed before the procedure. The bronchial lumen showed purulent bloody sputum from the right B2, without complete obstruction. After aspirating as much bloody sputum as possible, an EWS (Harada Co., Osaka, Japan) of M size was placed in the right B2a and that of L size in the right B1 and B2 using biopsy forceps. Bronchoscopy was performed again on day 7 of hospitalization since the L-sized EWS that had embolized the right B1 and B2 had retracted and was now located in the middle bronchial trunk. The M-sized EWS that occluded the right B2a had also migrated to the entrance of the right B2. The displaced L-sized EWS was retrieved using biopsy forceps. Furthermore, the M-sized EWS that had retracted was advanced into the right B2a using a bronchoscope. The B2b was completely open; nevertheless, an L-sized EWS was used to occlude it (Figure 3). Subsequently, hemostasis was achieved, and the patient was extubated on day 8 of hospitalization. However, severe hemoptysis recurred for the third time, and he required reintubation on day 10. Thereafter, 4 additional EBO+EWS procedures were performed (6 times in total). However, complete hemostasis was not achieved, and he died from hypoxemia due to hemoptysis on day 17 of hospitalization.
Discussion
In this case, the patient was treated with EBO+EWS after inadequate hemostasis with BAE; however, he did not achieve complete hemostasis and subsequently died. Hemoptysis is fatal; therefore, temporary hemostasis may provide a time delay that could be utilized as a bridge to other treatments.
EWS was originally developed for pneumothorax and tracheo-bronchial fistulas. However, its inventor, Dr. Watanabe, reported its possible effectiveness for hemoptysis [6]. In the treatment of pneumothorax, the EWS is inserted into the bronchus responsible for the air leak to achieve bronchial occlusion, which prevents airflow through the lung fistula and assists in closing the fistula (Figure 4A) [6]. In contrast, in the treatment of airway hemorrhage, the EWS is inserted into the responsible bleeding bronchus to stop the outflow of blood to other bronchi. In addition, the occluded bronchus fills with blood and coagulates, resulting in pressure hemostasis (Figure 4B) [12].
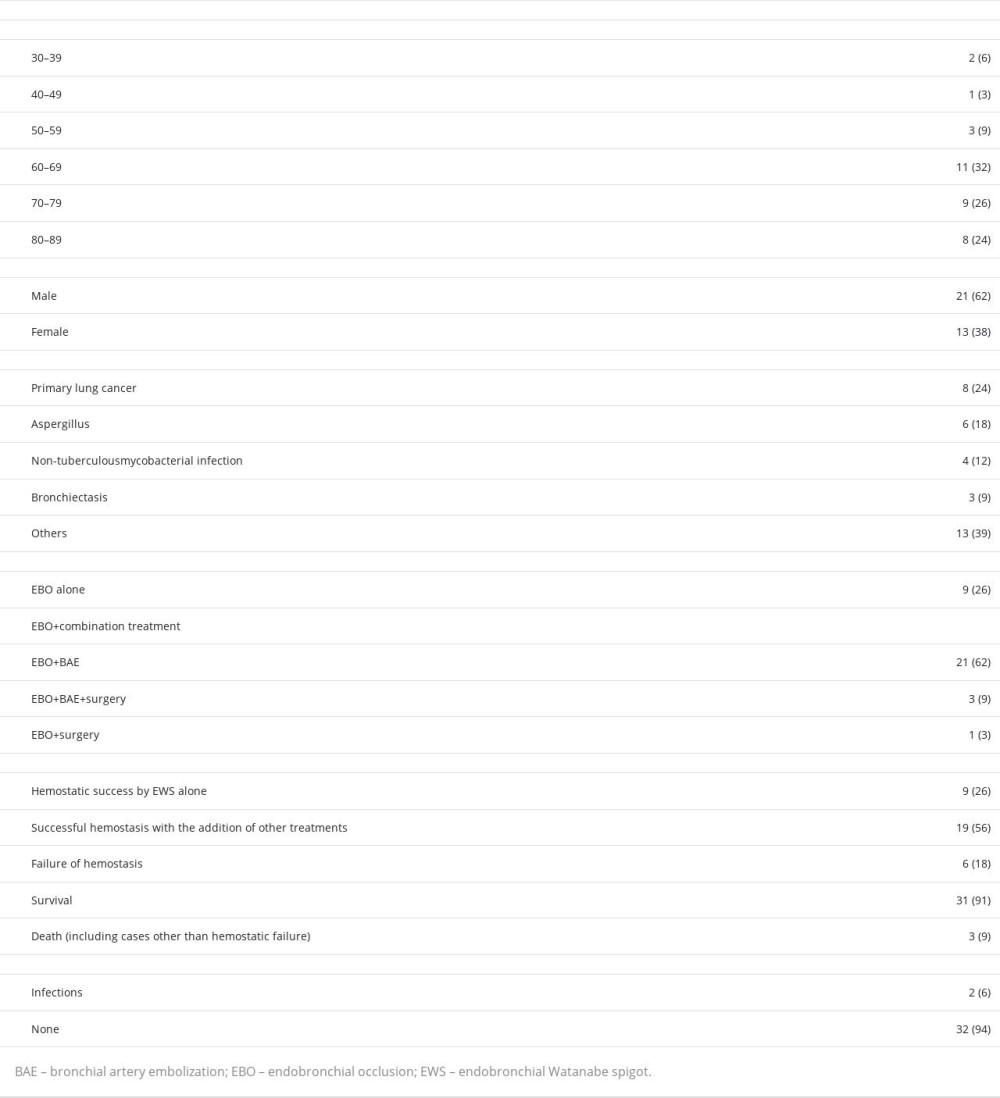
We were unable to achieve complete hemostasis in this case; however, temporary hemostasis was achieved with EBO+EWS. Previous studies have reported the effectiveness of EBO+EWS for hemostasis [5,7–27]. We analyzed 33 previously reported cases from 22 articles, in addition to our case. These are summarized in Table 1. Among the 34 cases, 17 (50%) were older than 70 years, 21 (62%) were men, and 13 (38%) were women. Primary lung cancer was the most common primary disease (
Similar to lung cancer, hemoptysis can recur in lung aspergillosis, even after successful hemostasis with BAE. Of the 34 patients, 6 had CPPA, including our patient. Hemostasis was achieved with EBO+EWS in 5 out of the 6 cases; however, it was not sustained in some cases. In our case, the patient already had advanced CPPA and numerous collateral hemorrhages, which may have contributed to the insufficient effect of BAE and EBO+EWS. In addition, the patient was already in an inoperable state owing to his poor pulmonary function and general condition, rendering him unsuitable for surgery. BAE and EBO+EWS were able to achieve temporary hemostasis; however, the hemoptysis could have caused asphyxia. Cases of uncontrolled hemoptysis, such as that in our patient, require attention. Successful control of hemoptysis with EBO+EWS may serve as a bridge to surgery or BAE, even with comorbid CPPA.
EBO+EWS can be effective for hemoptysis; however, our review has some limitations. The number of reported cases of hemoptysis management with EBO+EWS is limited; therefore, some publication bias may have occurred. Furthermore, we did not conduct a comparative analysis between EBO+EWS and alternative treatment approaches for hemoptysis. Additionally, the implementation of EBO+EWS for hemoptysis can be more difficult than EBO+EWS for pneumothorax because the bronchoscopic view in the former tends to be poor. Therefore, additional case studies should be conducted to explore the suitable applications of EBO+EWS in hemoptysis, particularly in patients with persistent hemostasis. Furthermore, future studies should consider comorbid conditions such as lung cancer and CPPA.
Conclusions
In this case, BAE and EBO+EWS were ineffective in controlling recurrent hemoptysis caused by CPPA. However, EBO+EWS can achieve temporary hemostasis, even in cases of severe hemoptysis. EBO+EWS can effectively control hemoptysis in appropriate cases, without the need for BAE or surgical lung resection. A multidisciplinary approach such as attempting hemostasis with combined EBO+EWS and BAE can serve as a treatment option in severe cases of hemoptysis. Prospective studies are required to explore the potential of EBO+EWS in hemoptysis management.
Figures
References:
1.. Yoon W, Kim JK, Kim YH, Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: A comprehensive review: Radiographics, 2002; 22(6); 1395-409
2.. Karlafti E, Tsavdaris D, Kotzakioulafi E, Which is the best way to treat massive hemoptysis? A systematic review and meta-analysis of observational studies: J Pers Med, 2023; 13(12); 1649
3.. Kettenbach J, Ittrich H, Gaubert JY, CIRSE standards of practice on bronchial artery embolisation: Cardiovasc Intervent Radiol, 2022; 45(6); 721-32
4.. Davidson K, Shojaee S, Managing massive hemoptysis: Chest, 2020; 157(1); 77-88
5.. Sakaguchi T, Kida H, Kanno Y, Bronchial occlusion with endobronchial watanabe spigot for hemoptysis in a mechanically ventilated patient with extracorporeal circulation: Intern Med, 2019; 58(2); 267-69
6.. Watanabe Y, Matsuo K, Tamaoki A, Bronchial occlusion with endo-bronchial Watanabe spigot: J Bronchol Interv Pulmonol, 2003; 10(4); 264
7.. Yagyu K, Miyamoto A, Matsushita H, Okimora A, A case of lung tumorlets secondary to pulmonary hypoplasia with recurrent haemoptysis: Respirol Case Rep, 2018; 6(8); e00373
8.. Hozumi T, Kajiura K, Nakamura K, Aorto-pleural fistula successfully treated by one-lung ventilation and wndobronchial Watanabe spigots: Respirol Case Rep, 2019; 7(1); e00382
9.. Taoka M, Makimoto G, Umakoshi N, Massive hemoptysis in a postoperative patient with recurrent lung cancer successfully treated by the combination therapy of endobronchial Watanabe spigot and bronchial artery embolization: Respir Med Case Rep, 2022; 38; 101669
10.. Oda N, Sakugawa M, Hosokawa S, Successful long-term management of two cases of moderate hemoptysis due to chronic cavitary pulmonary aspergillosis with bronchial occlusion using silicone spigots: Intern Med, 2018; 57(16); 2389-93
11.. Morikawa S, Okamura T, Minezawa T, A simple method of bronchial occlusion with silicone spigots (endobronchial Watanabe spigot; EWS®) using a curette: Ther Adv Respir Dis, 2016; 10(6); 518-24
12.. Adachi T, Ogawa K, Yamada N: Respir Investig, 2016; 54(2); 121-24
13.. Coiffard B, Laroumagne S, Plojoux J, Endobronchial occlusion for massive hemoptysis with a guidewire-assisted custom-made silicone spigot: A new technique: J Bronchol Interv Pulmonol, 2014; 21(4); 366
14.. Kawaguchi Y, Hanaoka J, Teramoto K, Pulmonary metastasis of invasive thymoma, showing endobronchial polypoid growth: Report of a case: Surg Today, 2014; 44(7); 1371-74
15.. Dutau H, Palot A, Haas A, Decamps I, Durieux O, Endobronchial embolization with a silicone spigot as a temporary treatment for massive hemoptysis: A new bronchoscopic approach of the disease: Respiration, 2006; 73(6); 830-32
16.. Bylicki O, Vandemoortele T, Laroumagne S, Temporary endobronchial embolization with silicone spigots for moderate hemoptysis: A retrospective study: Respiration, 2012; 84(3); 225-30
17.. Adachi T, Oki M, Saka H, Management considerations for the treatment of idiopathic massive hemoptysis with endobronchial occlusion combined with bronchial artery embolization: Intern Med, 2016; 55(2); 173-77
18.. Kho SS, Chan SK, Yong MC, Tie ST, Endobronchial embolization for life-threatening hemoptysis with endobronchial Watanabe spigot: BMC Res Notes, 2017; 10(1); 304
19.. Fujii A, Misumi Y, Hiyama J, [Case of intractable hemoptysis controlled by bronchial occlusion with an endobronchial Watanabe spigot (EWS).]: Nihon Kokyuki Gakkai Zasshi, 2008; 46(5); 416-19 [in Japanese]
20.. Nishihara T, Ishikawa H, Omachi N, Yamaguchi Y, A case of hemoptysis treated by combination therapy of bronchial artery embolization and an endo-bronchial Watanabe spigot: J Jpn Soc Respir Endosc, 2021; 43(6); 636-40
21.. Yaginuma H, Bronchial occlusion using endobronchial Watanabe spigot for massive hemoptysis in a case of pulmonary aspergilloma: J Jpn Soc Respir Endosc, 2017; 39(4); 349-53
22.. Ito K, Hataji O, Naito M, Effective control of bleeding with endobronchial Watanabe spigot (EWS) in two cases of massive hemoptysis after unsuccessful bronchial artery embolization (BAE): J Jpn Soc Respir Endosc, 2014; 36(2); 176-82
23.. Yoshiya T, Kanzaki M, Isaka T, A case of airway hemorrhage performed emergency hemostasis using endobronchial Watanabe spigot (EWS®): Nihon Rinsho Geka Gakkai Zasshi J Jpn Surg Assoc, 2013; 74(9); 2417-22
24.. Machida H, Shinohara T, Hatakeyama N, A case of lung aspergilloma in which endobronchial Watanabe spigot (EWS) was not effective for hemoptysis: J Jpn Soc Respir Endosc, 2012; 34(3); 262-66
25.. Sugahara M, Himeji D, Inoue Y, Successful treatment of refractory bronchial hemorrhage with bronchial occlusion using endobronchial Watanabe spigot: J Jpn Soc Respir Endosc, 2010; 32(1); 32-35
26.. Mizuno K, Fukai I, Endo K, A case of massive hemoptysis rescued by bronchial embolization after unsuccessful bronchial artery embolization: J Jpn Soc Respir Endosc, 2008; 30(6); 392-95
27.. Inada H, Furukawa K, Ishida J, Usefulness of endobronchial Watanabe spigot in the control for massive hemoptysis in a case of recurrent lung cancer: J Jpn Soc Respir Endosc, 2007; 29(2); 111-15
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250