25 April 2024: Articles 
Management of Nontraumatic Spontaneous Renal Hemorrhage (Wünderlich Syndrome) through Robotic-Assisted Laparoscopic Nephrectomy: A Case Series
Unusual or unexpected effect of treatment, Rare disease
Boju Tao1ACEF, Haoxun Zhang1B, Guoling Zhang1BD, Hua Liu1D, Le Meng1D, Xiangyu Zhu1F, Xuran Ji1F, Guang Jia1E, Ao Qi1E, Chunyang Wang1AEG*DOI: 10.12659/AJCR.942826
Am J Case Rep 2024; 25:e942826
Abstract
BACKGROUND: Wünderlich syndrome (WS) is a rare diagnosis of nontraumatic spontaneous renal hemorrhage into the subcapsular, perirenal, or pararenal spaces. Prompt and effective intervention is necessary for an accurate pathological diagnosis and preservation of life. In the current literature, open surgery is the primary option when conservative treatment fails, but there can be serious trauma and corresponding consequences. Herein, we present 3 cases of Wünderlich syndrome managed by robot-assisted laparoscopic nephrectomy via a retroperitoneal approach.
CASE REPORT: Patient 1 was a 44-year-old woman with right flank pain for 6 h. Patient 2 was a 53-year-old woman with a history of diabetes who had pain in her right flank pain and nausea for 1 day. Patient 3 was a 45-year-old man with left flank pain for 1 day. All cases of WS were confirmed by CT. All 3 patients were treated with retroperitoneal robot-assisted nephrectomy after conservative treatment failed. Pathological examination confirmed that patient 1 had angiomyolipoma, and patients 2 and 3 had renal clear cell carcinoma. At the 9-month follow-up, renal function was good and no evidence of recurrence or metastasis has been detected.
CONCLUSIONS: These cases have highlighted the importance of the clinical history and imaging findings in the diagnosis of Wünderlich syndrome, and show that rapid management can be achieved using robot-assisted laparoscopic nephrectomy. However, it is crucial to have a skilled surgical team and adequate preoperative preparation.
Keywords: Robotic Surgical Procedures, Kidney Diseases, Rupture, Spontaneous, Emergency Treatment
Introduction
Wünderlich syndrome (WS) is a rare and potentially fatal uro-logical emergency characterized by spontaneous, nontraumatic, acute renal hemorrhage extending into the subcapsular, perinephric, and/or pararenal space. In 1700, Bonet initially identified this symptom, while Wünderlich provided the first clinical description, referring to it as “spontaneous renal capsule apoplexy” in 1856 [1]. Coenen first used the term Wünderlich syndrome in 1910 [2].
The main clinical manifestation of WS is the Lenk triad, which includes sudden onset of lateral abdominal pain, a palpable lateral abdominal mass, and hypovolemic shock [3]. However, the simultaneous presence of all 3 is uncommon, occurring in only 20% of cases [4]. Abdominal pain is a common feature in almost all patients due to irritation of the retroperitoneal plexus and vomiting in some patients [5], but hematuria, flank mass, and hypovolemic shock can occur in isolation or in concert.
Tumor rupture, particularly in angiomyolipomas (AML) and renal cell carcinomas (RCC), is the primary cause of WS [6]. Non-tumor causes include infections, coagulation disorders, stone disease, and vascular factors, such as vasculitis, arteriovenous malformations, renal vein thrombosis, and renal aneurysms [7].
Cross-sectional imaging modalities such as CT, MRI, and ultra-sound have been used to evaluate patients who present with symptoms of WS [8]. CT is the recommended first step in the diagnostic process [9]. This technique is pivotal to detecting perirenal hemorrhage and identifying the underlying cause. It effectively detects the occurrence of perirenal hematoma, with a sensitivity of 92–100% [10].
The treatment of WS depends on whether the patient is hemodynamically stable [11]. Conservative treatment is recommended for hemodynamically stable patients with no evidence of ongoing bleeding, whereas open surgical treatment is the preferred approach in most cases for patients with persistent hemodynamic instability. Minimally invasive surgery, including laparoscopic and robotic approaches, has become the standard for routine nephrectomies because of its rapid development in the recent decades [12]. However, the applicability of these technologies during emergencies remains debatable. In 2011, Pena et al [13] reported the first case of laparoscopic treatment of ruptured angiomyolipoma (AML) during the acute bleeding phase. The transperitoneal approach was employed, with an operation time of 250 min and blood loss of 2000 mL (including hematoma).
Current robotic platforms can perform technically challenging procedures through minimally invasive means, including those traditionally performed using an open approach [14].
Compared with transperitoneal access, the retroperitoneal access provides direct access to the kidneys, minimizes manipulation of the intestines, and provides a clear anatomical view, speeding surgery [15]. However, to the best of our knowledge, there are no reports of robotic surgery in the emergency management of this rare disease.
This report presents 3 cases of Wünderlich syndrome managed by robot-assisted laparoscopic nephrectomy via a retroperitoneal approach. Our aim was to evaluate the safety and feasibility of a robot-assisted approach for rapid management of WS in emergency situations.
Case Reports
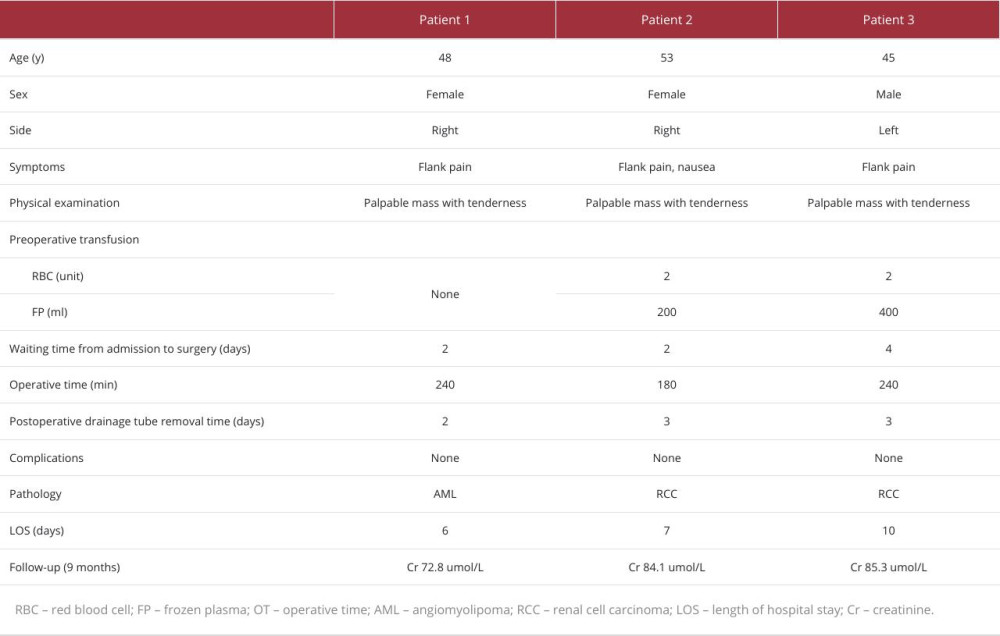
PATIENT 1:
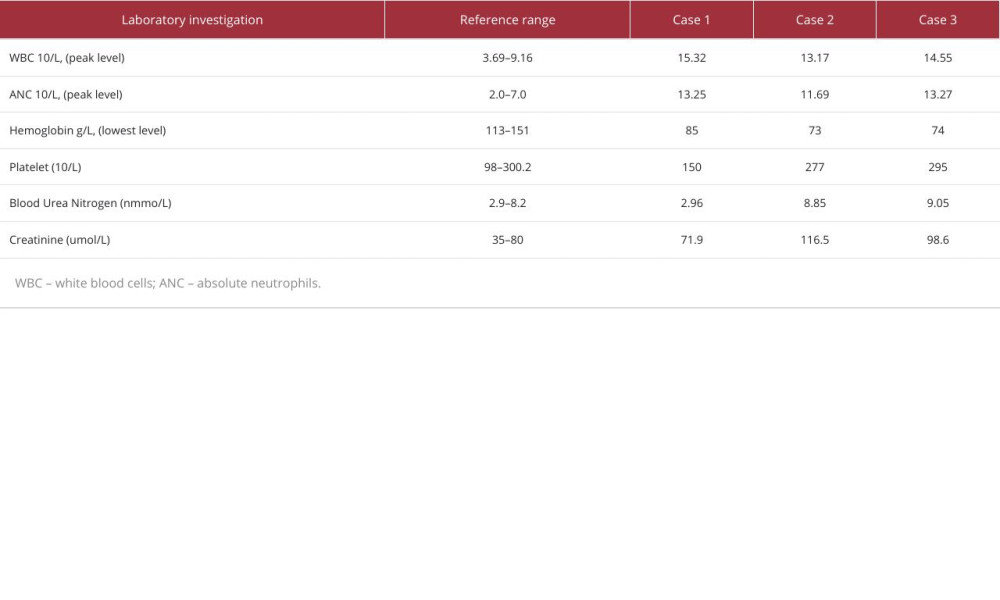
A 44-year-old woman presented to the Emergency Department (ED) with sudden onset of severe right-side flank pain of 6-h duration (Table 1). She denied nausea, vomiting, or fever at that time. The patient has no history of previous trauma, surgery, or comorbidities. The physical examination noted a palpable mass at the right flank and tenderness in the right flank. Contrast-enhanced CT scan (Figure 1) revealed a heterogeneous density shadow in the right kidney, with portions extending beyond the renal parenchyma. The internal density was uneven, exhibiting both fat-density and soft-tissue density shadows. Upon contrast enhancement, the lesion demonstrated uneven moderate enhancement. The laboratory tests (Table 2) showed elevated white blood cells and decreased hemoglobin (111 g/L). After admission, the patient was given conservative treatment such as hemostasis, rehydration, and anti-inflammatory therapy. On the second day of conservative treatment, the patient’s hemoglobin dropped from 111 g/L to 85 g/L and intermittent hematuria occurred. Since the disease was progressing and the patient and her family wanted surgical treatment, we decided to perform the surgery. The operation duration was 240 min, and a transfusion of 4 units of red blood cells and 200 ml of frozen plasma were performed during the operation. The pathological diagnosis was angiomyolipoma.
PATIENT 2:
A 53-year-old woman came to the ED with sudden onset of right-sided flank pain and nausea for 1 day (Table 1). She had a medical history of diabetes mellitus and a surgical history of cesarean section, with no history of trauma. Contrast-enhanced CT (Figure 2) revealed an altered contour of the right kidney, accompanied by slightly increased density shadows in the perirenal area. The right kidney displayed a lesion with a roughly circular mixed density, extending outward and possessing unclear margins. After administration of contrast agent, the lesion showed uneven enhancement, with significant enhancement during the cortical phase, and the lesion’s density was lower than that of the renal parenchyma during the excretory phase. The laboratory tests (Table 2) showed elevated white blood cells and decreased hemoglobin (78 g/L). After admission, she received conservative treatment and was given 2 units of red blood cells and 200 ml of frozen plasma. However, there was no improvement and her hemoglobin continued to decrease (73 g/L). Surgical management was chosen. The duration of the procedure from the time of anesthesia to the conclusion of the procedure was approximately 180 min. Intraoperatively, 8 units of red blood cells and 200 ml of frozen plasma were transfused. The pathological diagnosis was clear cell RCC.
PATIENT 3:
A 45-year-old man presented to the ED due to persistent worsening pain in the left flank, which began 1 day earlier, without any apparent precipitating factors (Table 1). The patient denied experiencing symptoms such as nausea, vomiting, or fever. He also reported no history of trauma, previous surgeries, or comorbidities. Physical examination found percussion pain in the left kidney area. Contrast-enhanced CT imaging (Figure 3) revealed a quasi-circular mixed-density shadow at the location of the left renal pelvis, with a crescent-shaped high-density shadow visible along the margin of the left kidney. Additionally, irregular high-density shadows and striated low-density shadows were observed around the left kidney and in the retroperitoneal space. The laboratory tests (Table 2) showed elevated white blood cells and decreased hemoglobin (125 g/L). During conservative treatment after admission, his hemoglobin continued to decline. The hemoglobin dropped on day 3 of admission to 74 g/L. After transfusing 2 units of red blood cells and 400 ml of plasma, there was no significant increase in hemoglobin (76 g/L). Further surgery was chosen to clarify the diagnosis and treatment. The total time of surgery and anesthesia was 240 min. The patient was transfused intraoperatively with 8 units of red blood cells and 200 ml of plasma. The pathological diagnosis was clear cell RCC.
PORT LOCATION AND SURGICAL APPROACH: After general anesthesia, the patient was positioned in the healthy lateral decubitus position with a raised lumbar bridge and was secured to the operating table. The trocar placement mirrored radical nephrectomy (Figure 4). After the extraperitoneal fat was fully removed, the Gerota’s fascia was opened, and many peri-renal hematomas, broken pieces of kidney embedded in the hematoma, and adipose tissue were observed. First, we cleared the blood clots and improved the surgical field of view. To identify anatomical structures, the blood clots were continuously aspirated using an aspirator. The renal arteries are then controlled, and careful dissection techniques were used to ensure full exposure of the renal arteries, given the anatomical displacement caused by renal rupture and hematoma compression. The left and right renal pedicles were identified using distinct anatomical landmarks.
On the right side (patients 1 and 2), the inferior vena cava was observed and used as an effective anatomical marker to locate and identify the hilus of the kidney (Figure 5). Dissection was performed along the inferior vena cava toward the medial middle of the kidney. The right renal artery is usually reached when the inferior vena cava is invisible. The pulsating renal artery was observed after the surrounding connective tissue was opened. The right renal artery was clamped and ligated using Hem-o-Lok clips (Video 1). Subsequently, dissection progressed anteriorly along the inferior vena cava to locate the right renal vein. When freeing the right renal vein, attention was paid to fully expose the upper and lower angles of the junction with the inferior vena cava to prevent damage to the latter (Video 2).
On the left side (patient 3), the kidney was gently pushed ventrally after fully freeing it laterally and dorsally. A localized bulge in the middle of the dorsal medial aspect of the kidney indicated presence of a renal artery (Figure 6). Further dissection was performed along this area, and the connective tissue was carefully opened to reveal pulsations in the renal artery. The left renal artery was clamped and ligated using Hem-o-Lok clamps. Because the subphrenic, central adrenal, lumbar, and genital veins converge in the left renal vein, blind separation can damage these small veins and increase bleeding, particularly in cases of anatomical variations and unclear vision. First, we freed the ventral side of the kidney from top to bottom outside the fat capsule, and then turned the kidney over. The kidney was tractioned, and the left renal vein was found at the position of the connection between the kidney and the inferior vena cava.
After the nephrectomy was completed, the wound cavity was repeatedly soaked in distilled water. Finally, a drainage tube was placed in the retroperitoneal cavity, and the wound was stitched layer-by-layer.
Discussion
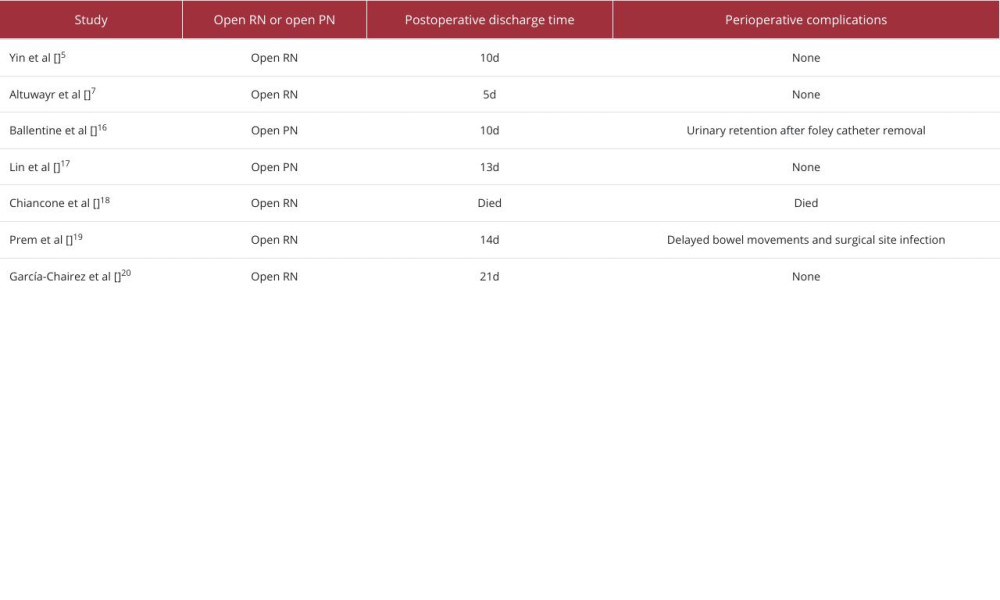
These 3 cases illustrate the potential of robot-assisted therapy as a therapeutic option for patients with WS. Three patients underwent retroperitoneal robot-assisted treatment, and the decision was ultimately made to proceed with radical resection because extensive and irreparable parenchymal damage with ongoing bleeding was observed intraoperatively in all patients. Postoperative recovery was rapid, with no complications or adverse reactions. Compared to traditional open surgery, these 3 patients had significantly shorter incision lengths (Figure 7) and hospitalization times (Table 3). Contrast-enhanced CT was used as the main examination to confirm the diagnosis, as non-enhanced CT cannot clarify the underlying cause of bleeding. Contrast-enhanced CT helps differentiate the various causes of kidney rupture and the extent of bleeding [21]. When patients have contraindications to contrast-enhanced CT, such as allergy to contrast agents or renal insufficiency, magnetic resonance imaging (MRI) can be used as an alternative to detect small renal tumors after the acute bleeding phase has subsided [22] and to detect hemorrhagic cysts [23]. However, MRI cannot be widely used in emergencies because of its long scan times and low utilization rates. Ultrasound is a quick, safe, and inexpensive method for diagnosing diseases. Although it is not suitable for determining the etiology, it can be used as a follow-up method to assess the recovery of hematoma [24].
The primary management strategies for WS include conservative treatment, vascular embolization, and surgery (radical or partial nephrectomy). In patients with stable vital signs and no detectable malignancy or large AML on the initial CT scan, conservative management with close monitoring can be used [23,24], but it cannot identify the cause of the disease, which can cause patient anxiety. Additionally, renal function should be closely monitored during follow-up. If the split renal function is completely impaired, radical nephrectomy should be performed in a timely manner to prevent further complications caused by necrotic organs [25]. Vascular embolization can prevent acute and life-threatening bleeding while protecting the renal parenchyma [21,26]. However, due to the inflammatory response to necrotic tissue after embolization, patients may develop post-embolization syndromes, including abscesses, pleural effusion, lumbar pain, fever, leukocytosis, and nausea [27]. In addition, there is a significant risk of recurrent bleeding and tumor recurrence. It has been reported that 30% of patients treated with embolization undergo re-embolization or subsequent surgery [28]. Moreover, embolization aimed at delaying surgery can complicate subsequent surgeries and affect the clinical stage of the disease [22].
In our case series, all 3 patients initially received conservative treatment, but their hemoglobin levels continued to fall despite treatment. Therefore, the decision was made to proceed with surgical intervention. Considering the longer incisions and greater trauma associated with open surgery, we opted to attempt the procedure with robot assistance. The key points of such operations are to remove blood clots and improve the visual field. Bleeding was effectively controlled using the pressure induced by the robot’s pneumoperitoneum, while blood clots and fragmented fat tissue were continuously suctioned through the aspirator. If continuous bleeding occurs, a gauze can be used for tamponade. In conventional retroperitoneal laparoscopic radical nephrectomy, the renal pedicle is typically positioned via the psoas muscle, arcuate ligament, and diaphragm [29]. However, finding the arcuate ligament in our 3 patients was relatively difficult because of the anatomical displacement and the relatively limited field of view caused by renal parenchymal rupture and hemorrhage. In this complex situation, the advantages of 3-dimensional high-definition magnified field of view and flexible and bendable robot arm provided by the robot system may surpass those of laparoscopic and open surgery [30]. Our approach involved initial lateral and dorsal dissection of the kidney and pushing it ventrally to create more space in the surgical field. On the left side, raised connective tissue was observed in the middle of the dorsal medial part of the kidney, allowing access to the left renal artery after dissection through the connective tissue. The right renal artery can be identified by dissection along the inferior vena cava until it is no longer visible.
Conventionally, the transperitoneal approach provides more space and facilitates easier operations [31]. However, once the lateral and dorsal aspects of the kidney are completely freed and pushed ventrally, the retroperitoneal space becomes comparable in size to the transperitoneal space. The primary advantage of the retroperitoneal approach is direct access to the renal artery [32], which is crucial when there is persistent renal bleeding. Furthermore, the retroperitoneal approach minimizes the risk of abdominal organ damage and abdominal adhesions and accelerates the postoperative recovery of intestinal function [33]. Notably, surgical field metastases have been reported after laparoscopic transperitoneal partial nephrectomy in patients with WS due to renal cell carcinoma [34]. Therefore, when treating ruptured and bleeding renal cell carcinoma, the retroperitoneal approach can help prevent peritoneal dissemination. In our 3 patients, the retroperitoneal cavity was thoroughly irrigated with distilled water after kidney removal to prevent tumor spread.
To the best of our knowledge, our study is the first report use of retroperitoneal robot-assisted surgery for WS, even in the acute phase of bleeding, with the aim of exploring its application potential. Although our data demonstrated the benefits of retroperitoneal robot-assisted surgery in the treatment of WS, revealing its safety and feasibility as a valuable alternative treatment, some apparent limitations should not be ignored. Owing to its relatively rare incidence, we had a rather small number of cases, and no controlled studies could be performed. Therefore, well-designed prospective randomized controlled studies are required in the future to confirm the feasibility of this technique. It is important to emphasize that if a patient is already in shock, emergency open surgery remains the recommended course of action to save the patient’s life.
Conclusions
These 3 cases have highlighted the importance of the clinical history and imaging findings in the diagnosis of Wünderlich syndrome, and show that rapid management can be achieved using robot-assisted laparoscopic nephrectomy.
Figures
References:
1.. Daliakopoulos SI, Spontaneous retroperitoneal hematoma: A rare devastating clinical entity of a pleiada of less common origins: J Surg Tech Case Rep, 2011; 3(1); 8-9
2.. Albi G, del Campo L, Tagarro D, Wünderlich’s syndrome: Xauses, diagnosis and radiological management: Clin Radiol, 2002; 57(9); 840-45
3.. Simkins A, Maiti A, Cherian SV, Wünderlich syndrome: Am J Med, 2017; 130(5); e217-18
4.. Grubb SM, Stuart JI, Harper HM, Sudden onset flank pain: Spontaneous renal rupture: Am J Emerg Med, 2017; 35(11); 1787.e1-e3
5.. Yin G, Pan X, Tian H, Spontaneous renal rupture due to renal calculi: A case report and literature review: Exp Ther Med, 2022; 24(3); 588
6.. Mao Y, De Oliveira IS, Hedgire S, Aetiology, imaging features, and evolution of spontaneous perirenal haemorrhage: Clin Radiol, 2017; 72(2); 175.e19-e26
7.. Altuwayr RM, Almutairi FS, Alkhaibari SH, Spontaneous rupture of large angiomyolipoma of the kidney: A rare case: Cureus, 2021; 13(11); e19908
8.. Diaz JR, Agriantonis DJ, Aguila J, Spontaneous perirenal hemorrhage: What radiologists need to know: Emerg Radiol, 2011; 18(4); 329-34
9.. Kim JW, Kim JY, Ahn ST, Spontaneous perirenal hemorrhage (Wünderlich syndrome): An analysis of 28 cases: Am J Emerg Med, 2019; 37(1); 45-47
10.. Parameswaran B, Khalid M, Malik N, Wünderlich syndrome following rupture of a renal angiomyolipoma: Ann Saudi Med, 2006; 26(4); 310-12
11.. Ahn T, Roberts MJ, Navaratnam A, Changing etiology and management patterns for spontaneous renal hemorrhage: A systematic review of contemporary series: Int Urol Nephrol, 2017; 49(11); 1897-905
12.. Golombos DM, Chughtai B, Trinh QD, Minimally invasive vs open nephrectomy in the modern era: Does approach matter?: World J Urol, 2017; 35(10); 1557-68
13.. Peña JA, Serrano M, Cosentino M, Laparoscopic management of spontaneous retroperitoneal hemorrhage: Urol Int, 2011; 87(1); 114-16
14.. Lee CU, Alabbasi M, Chung JH, How far has robot-assisted partial nephrectomy reached?: Investig Clin Urol, 2023; 64(5); 435-47
15.. Paciotti M, Piramide F, Bravi CA, Retroperitoneal approach for robot-assisted partial nephrectomy: Still underused despite the supporting evidence: Minerva Urol Nephrol, 2023; 75(5); 652-55
16.. Ballentine WK, Vilson F, Dyer RB, Mirzazadeh M, Nephron-sparing management of Xanthogranulomatous pyelonephritis presenting as spontaneous renal hemorrhage: A case report and literature review: BMC Urol, 2018; 18(1); 57
17.. Lin YY, Hsu CW, Li HM, Su HY, Diagnosis of Wünderlich syndrome in a patient with flank pain: Hong Kong Med J, 2019; 25(5); 406 e1–e2
18.. Chiancone F, Meccariello C, Ferraiuolo M, A rare case of spontaneous parenchymal kidney explosion in a patient with ureteral obstruction caused by a single stone: Urologia, 2021; 88(4); 386-88
19.. Prem K, Smita S, Pankaj K, Pragya P, Surgical management of spontaneously ruptured kidney with peritonitis due to neglected renal and ureteric calculi: BMJ Case Rep, 2021; 14(6); e240910
20.. García-Chairez LR, Montelongo-Rodríguez FA, Moreno-Arquieta IA, Unusual presentation of Wünderlich syndrome: Ochsner J, 2022; 22(3); 273-76
21.. Ho TH, Yang FC, Cheng KC, Wünderlich syndrome, spontaneous ruptured renal angiomyolipoma and tuberous sclerosis: QJM, 2019; 112(4); 283-84
22.. Aldughiman AW, Alsunbul A, Al-Gadheeb A, Does spontaneous renal hemorrhage mandate close surveillance for impending renal cell carcinoma? A case report and literature review: Int J Surg Case Rep, 2020; 73; 44-47
23.. Sirajudeen J, Purayil NK, Parambath A, Kayakkool M, A renal colic mimic – Wünderlich syndrome: A case report: Cureus, 2020; 12(10); e11242
24.. Larbi H, Hassan I, Cherraqi A, Wünderlich syndrome: Rare and unrecognized emergency: Urol Case Rep, 2022; 43; 102093
25.. Mariolis-Sapsakos T, Nannou E, Angelis S, Filippou D, Wünderlich syndrome: Spontaneous cystic rupture on account of acquired kidney atrophy: Cureus, 2022; 14(10); e30386
26.. Choi HS, Kim CS, Ma SK, Wünderlich syndrome and regression of angiomyolipoma: Korean J Intern Med, 2020; 35(6); 1528-29
27.. Halpenny D, Snow A, McNeill G, Torreggiani WC, The radiological diagnosis and treatment of renal angiomyolipoma current status: Clin Radiol, 2010; 65(2); 99-108
28.. Fernández-Pello S, Hora M, Kuusk T, Management of sporadic renal angiomyolipomas: A systematic review of available evidence to guide recommendations from the European Association of Urology Renal Cell Carcinoma Guidelines Panel: Eur Urol Oncol, 2020; 3(1); 57-72
29.. Shi L, Cai W, Dong J, A novel approach to locate renal artery during retroperitoneal laparoendoscopic single-site radical nephrectomy: Int J Clin Exp Med, 2014; 7(7); 1752-56
30.. Ashrafi AN, Gill IS, Minimally invasive radical nephrectomy: A contemporary review: Transl Androl Urol, 2020; 9(6); 3112-22
31.. Garg M, Singh V, Sinha RJ, Sharma P, Prospective randomized comparison of transperitoneal vs retroperitoneal laparoscopic simple nephrectomy: Urology, 2014; 84(2); 335-39
32.. Desai MM, Strzempkowski B, Matin SF, Prospective randomized comparison of transperitoneal versus retroperitoneal laparoscopic radical nephrectomy: J Urol, 2005; 173(1); 38-41
33.. Porreca A, D’Agostino D, Dente D, Retroperitoneal approach for robot-assisted partial nephrectomy: Technique and early outcomes: Int Braz J Urol, 2018; 44(1); 63-68
34.. Castillo OA, Vitagliano G, Díaz M, Sánchez-Salas R, Port-site metastasis after laparoscopic partial nephrectomy: Case report and literature review: J Endourol, 2007; 21(4); 404-7
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250