06 July 2020: Articles 
Nesidioblastosis Associated with Pancreatic Heterotopia as a Differential Diagnosis of Hypoglycemia: A Literature Review and Case Report
Challenging differential diagnosis, Rare coexistence of disease or pathology
Aline A. Lopes1ABCDEF*, Ana C. Miranda1BE, Marcelo S. Maior2BE, Roberto V. de Mello2BE, Francisco A. Bandeira1ABCDEFDOI: 10.12659/AJCR.922778
Am J Case Rep 2020; 21:e922778
Abstract
BACKGROUND: Nesidioblastosis is a rare disease that is part of the differential diagnosis of pancreatogenic hyperinsulinemic hypoglycemia (PHH) in patients whose imaging studies do not localize insulinoma. Pancreatic heterotopia is a rare congenital abnormality characterized by pancreatic tissue anatomically separated from the main gland and found in 0.5% of abdominal surgeries. The purpose of this article is to provide a systematic review of the literature on nesidioblastosis in pancreatic ectopic tissue and to describe a case of the co-occurrence of these 2 rare conditions.
CASE REPORT: A 32-year-old man presented with adrenergic and neuroglycopenic symptoms, with laboratory-confirmed hyperinsulinemic hypoglycemia. There was no evidence of tumors on abdominal CT scan and MRI. Celiac trunk sampling with a calcium stimulation test was done, which showed an insulin gradient in the gastroduodenal artery. However, the intraoperative ultrasound showed a small nodule located at the pancreatic tail, leading to distal pancreatectomy. The histologic examination showed nesidioblastosis associated with pancreatic heterotopia. The patient remained asymptomatic after distal pancreatectomy.
CONCLUSIONS: Nesidioblastosis accounts for 0.5%–5% of all cases of PHH, with a histology showing hypertrophy and hyperplasia of pancreatic islets. Pancreatic heterotopia is a rare congenital anomaly resulting from failure of pancreatic cell migration, and is found as an incidentaloma in imaging or surgeries. Although it is a rare disease, nesidioblastosis should be considered in the investigation of hypoglycemia, even in the rare presentation of nesidioblastosis in patients with pancreatic heterotopy.
Keywords: Congenital Hyperinsulinism, Hypoglycemia, Islets of Langerhans, Nesidioblastosis, Choristoma, Diagnosis, Differential, Pancreas, Pancreatectomy
Background
Hypoglycemia is uncommon in patients who do not receive hypoglycemic treatment, and patients who present Whipple’s triad should receive additional investigation for hypoglycemia [1]. In adults, insulinoma is the most frequent cause of pancreatogenic hyperinsulinemic hypoglycemia (PHH) [2]; nesidioblastosis, although more common in children, accounts for 0.5–5% of cases of PHH [3], or 0.09% per 100 000 population/year in a Japanese survey [2]. Non-insulinoma pancreatogenous hypoglycemia syndrome (NIPHS) is characterized by typical histologic findings and should be considered when imaging tests are negative for localization of insulinoma, with positive selective arterial calcium stimulation test results [4].
For these patients, selective arterial calcium stimulation testing with hepatic venous sampling (SACST) should be done [5], in which an elevation of insulin after calcium stimulation may suggest pancreatic areas of higher insulin production [5]. This exam is essential when scans with 111In or 68Galio (SPECT/CT or scintigraphy) are not available; as well as endoscopic US, which is an invasive method with limitations to visualize lesion in the pancreas tail [6]. In NIPHS, a positive response may occur in more than 1 pancreatic region or in multiple arteries, suggesting diffuse hyperplasia [7].
Nesidioblastosis, first described in 1938 by Laidlaw [8], is a rare disease in adults and is part of the diagnostic spectrum of NIPHS, which is characterized by typical histologic findings: hypertrophy and/or hyperplasia of pancreatic islets, enlarged and hyperchromatic nuclei, and neoformation of pancreatic islets from the duct epithelium [9].
Nesidioblastosis is believed to be genetically determined, inserted in the context of PHH syndromes. Currently, 11 genes involved in PHH are known: ABCC8, KCNJ11, HNF4A, HNF1A, GLUD1, GCK, HADH1, UCP2, MCT1, HK1, and PGM1; in addition to genetic syndromes such as Turner and Beckwith-Wiedemann syndromes [10], with different patterns of inheritance. There is also a description of innate metabolism errors, with hypoglycemias since childhood, such as glycogen storage disease, activating mutations of monocarboxylate transporter-1, and deficiency of glucose transporter-2 [11].
In contrast, pancreatic heterotopia is characterized by a congenital abnormality in which pancreatic tissue is anatomically separated from the main gland, corresponding to an ectopic, heterotopic, or accessory pancreas, and it usually occurs in the gastrointestinal tract [12]. The actual incidence of heterotopic pancreas is unknown, being an incidental finding in autopsies, surgeries, or imaging tests, with a prevalence of approximately 0.5–13.7% in autopsies [13]. The present study aims to provide a systematic review on the occurrence of nesidioblastosis in ectopic pancreatic tissue, and to report an additional case of the coexistence of these 2 conditions.
Case Report
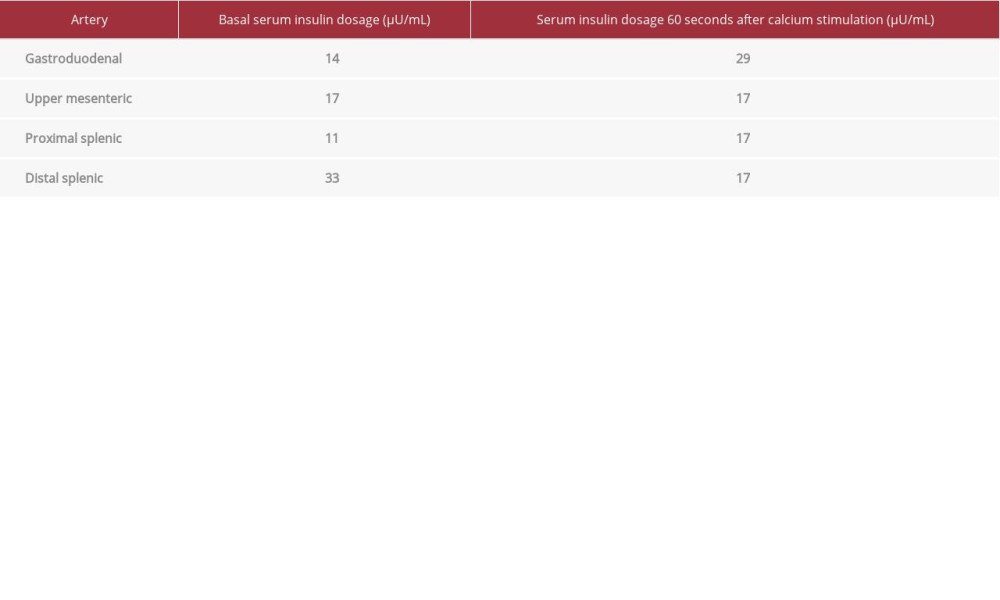
A 32-year-old man presented with visual turbidity, tachycardia, tremors, and cold sweating after physical exertion, which began 5 months ago. Subsequently, the symptoms began to appear at rest. The patient also reported admission to the emergency room with hypoglycemia symptoms, confirmed by capillary blood glucose measurements. The patient had no previous diseases, including diabetes mellitus (DM), no previous use of hypoglycemic agents such as sulphonylurea or insulin, and no history of abdominal surgery. In the patient’s family history, there were no cases of DM nor hypoglycemia. The patient was admitted at our hospital. During hospitalization, the physical examination showed a good general condition, with normal neurologic, respiratory, and cardiovascular results. Capillary glucose monitoring confirmed repeated hypoglycemia episodes fasting and postprandial. We performed a prolonged fasting test with constant measurements of capillary glucose, and after approximately 145 minutes, he presented hypoglycemia. The plasma glucose, c-peptide, and insulin levels during hypoglycemic symptoms were 48 mg/dL, 2.3 ng/mL and 16.9 μU/mL, respectively. The serum cortisol during hypoglycemia was 21 mcg/dL. Unfortunately, we did not have sulphonylurea metabolite screening or anti-insulin antibody dosage available. Considering that insulinoma would be the most common cause of hypoglycemia, the patient underwent exams to locate the lesion. The abdominal tomography scan and magnetic resonance image (MRI) showed no evidence of a pancreatic tumor (Figure 1A, 1B). A SACST was done after celiac trunk catheterization, showing the niche with increased vascularization (Table 1, Figure 1C).
On October 2017, the patient underwent open surgery, but no tumors were found in the pancreatic head. However, the intra-operative ultrasound showed a small 9-mm nodule (Figure 1D), which was resected along with the distal part of the pancreas. The histologic examination showed a heterotopic pancreas associated with nesidioblastosis, in the topic and ectopic pancreas (Figure 2). Postoperatively, there were no further episodes of hypoglycemia, and the patient remained asymptomatic.
Discussion
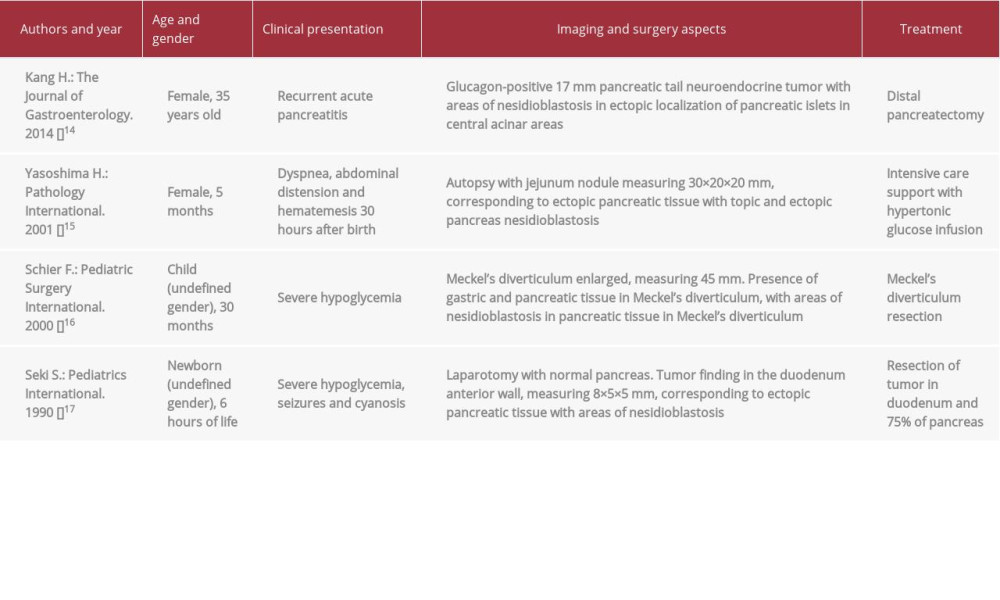
We performed a systematic literature review using the terms “heterotopic pancreas and nesidioblastosis” in the PubMed database to search for reports published, without restriction of time of publication. A total of 7 reports were found. When using the descriptors “ectopic pancreas and nesidioblastosis”, 3 more articles were found. Of a total of 10 articles, 6 were excluded because they did not have the coexistence of the 2 conditions. In all, 4 articles remained, the main aspects of which are described in Table 2.
In an epidemiological survey conducted in Japan between 2017 and 2018, the estimated prevalence of NIPHS was 0.09% per 100 00 adult population, with 33 patients with NIPHS identified, of which only 10 had nesidioblastosis that developed in adulthood [2].
In the present case, there was an insulin gradient in the gastroduodenal artery, suggesting that the lesion was in the head of the pancreas. Figure 1C shows a niche with increased vascularization in the area of hyperinsulinism in SACST. However, during surgery, the intraoperative US plus pancreatic exploration showed a tail lesion and normal pancreatic head. This was probably due to the multifocal lesion aspects of nesidioblastosis, justifying a positive SACST for the pancreas head, where islets were highly responsive, but with no characteristics of a true nodule, and they were not responsible for the hypoglycemic episodes.
Disagreement between preoperative location exams may occur. Wild et al. reported a patient whose SACST suggested a location of lesion in the territory of the superior mesenteric artery, but when a molecular method was used (GLP1-receptor Scan), it found a lesion in the mesentery, below the uncinated process [18]. This disagreement between the test directly affects therapeutic decision-making and surgical proposal, similar to the present case.
Another helpful imaging exam is DOPA-PET. Luo et al. found sensitivity higher than 97% in 45 patients with PHH with 68Ga-PET/CT [19], as well as high sensitivity in adult nesidioblastosis and congenital hyperinsulinism [10]. Another study showed that the accuracy of 68Ga-DOTA-exendin-4 PET/CT was 93.9%, considerably higher than SPECT/CT (67.5%) and MRI (67.6%) [20]. Despite the high accuracy, this method is expensive and not widely available, being still restricted to research centers, and it is not used routinely.
Similar to the care reported by Kang et al. [14], our patient was an adult, with less severe condition and resolution after surgery; unlike when the disease occurs in childhood, with symptoms of early onset and ectopia at different points in the gastrointestinal tract. Due to the low prevalence and rarity of coexistence of these 2 conditions, it is unlikely that children present more severe conditions than adults.
Although our patient had total control of hypoglycemia after surgery, it is possible that future recurrence may take place and that the observed gradient at the pancreatic head may represent a temporary silent pancreatic hyperplasia. Martin-Grace et al. [21] reported hypoglycemia recurrence 6 weeks after distal pancreatectomy, achieving good glycemic control with clinical treatment. Yamada et al. found 40% residual hypoglycemia after 5 years of follow-up, with 2 patients with neurological sequalae [2].
According to the literature, the main treatment consists of surgery, usually distal pancreatectomy [22]. As a complementary clinical treatment, there are descriptions of the use of glucocorticoids, calcium channel blockers, diazoxide [23], octreo-tide, pasireotide [24], and acarbose [2].
Pancreatic heterotopia, also called an ectopic or accessory pancreas, involves pancreatic tissue anatomically separated from the main pancreatic gland, without continuity by vessels or ducts, as described by Rezvani et al. [25]. In this case, there was a strip of adipose tissue, seen only under microscopy, separating the main pancreatic gland from a second pancreatic tissue, as an accessory pancreas. It is worth noting that nesidioblastosis foci existed in both areas – the main and the ectopic pancreas – contributing to the presented hypoglycemia.
Pancreatic ectopia is a congenital abnormality. The cause is unknown, although migration failure is believed to occur with fragments of pancreatic tissue dispersed throughout the gastrointestinal tract during embryogenesis, developing into an isolated topography of the main pancreatic gland [26]. This tissue, although ectopic, has the same risk of topic pancreas to development of benign and malign lesion, with reports of pancreatic adenocarcinoma and intraepithelial neoplasm [27].
Conclusions
The presence of nesidioblastosis in ectopic pancreatic tissue is quite rare, as evidenced in the literature review. Although nesidioblastosis is a rare disease, it should be part of the differential diagnosis in patients with hypoglycemia, especially when imaging is negative for insulinoma and pancreatic heterotopy.
Figures
References:
1.. Cryer PE, Axelrod L, Grossman AB, Evaluation and management of adult hypoglycemic disorders: An Endocrine Society Clinical Practice Guideline: J Clin Endocrinol Metab, 2009; 94(3); 709-28
2.. Yamada Y, Kitayama K, Oyachi M, Nationwide survey of endogenous hyperinsulinemic hypoglycemia in Japan 2017–2018 : Congenital hyper-insulinism, insulinoma, non-insulinoma pancreatogenous hypoglycemia syndrome and insulin autoimmune syndrome (Hirata’s disease): J Diabetes Investig, 2019 [Epub ahead of print]
3.. Van der Wal BC, De Krijger RR, De Herder WW, Adult hyperinsulinemic hypoglycemia not caused by an insulinoma: A report of two cases: Virchows Archiv, 2000; 436(5); 481-86
4.. Anderson B, Nostedt J, Girgis S, Insulinoma or non-insulinoma pancreatogenous hypoglycemia? A diagnostic dilemma: J Surg Case Rep, 2016; 2016(11); rjw188 pii:
5.. Falconi M, Eriksson B, Kaltsas G, ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors: Neuroendocrinology, 2016; 103(2); 153-71
6.. Christ E, Antwi K, Fani M, Wild D, Innovative imaging of insulinoma: The end of sampling? A review: Endocr Relat Cancer, 2020; 27(4); R79-92
7.. Thompson SM, Vella A, Thompson GB, Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis: J Clin Endocrinol Metab, 2015; 100(11); 4189-97
8.. Laidlaw GF, Nesidioblastoma, the islet tumor of the pancreas: Am L Pathol, 1938; 14(2); 125
9.. Anlauf M, Wieben D, Perren A, Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: Diagnostic criteria, incidence, and characterization of β-cell changes: Am J Surg Pathol, 2005; 29(4); 524-33
10.. Stanley CA, Perspective on the genetics and diagnosis of congenital hyper-insulinism disorders: J Clin Endocrinol Metab, 2016; 101(3); 815-26
11.. Douillard C, Mention K, Dobbelaere D, Hypoglycaemia related to inherited metabolic diseases in adults: Orphanet J Rare Dis, 2012; 7(1); 26
12.. Kung JW, Brown A, Kruskal JB, Heterotopic pancreas: Typical and atypical imaging findings: Clin Radiol, 2010; 65(5); 403-7
13.. Wei R, Wang QB, Chen QH, Upper gastrointestinal tract heterotopic pancreas: Findings from CT and endoscopic imaging with histopathologic correlation: Clin Imaging, 2011; 35(5); 353-59
14.. Kang H, Kim S, Lim TS, [A case of alpha-cell nesidioblastosis and hyperplasia with multiple glucagon-producing endocrine cell tumor of the pancreas]: Korean J Gastroenterol, 2014; 63(4); 253-57 [in Korean]
15.. Yasoshima H, Nakata Y, Ohkubo E, An autopsy case of pancreatic and ectopic nesidioblastosis: Pathol Int, 2001; 51(5); 376-79
16.. Schier F, Sauerbrey A, Kosmehl H, A Meckel’s diverticulum containing pancreatic tissue and nesidioblastosis in a patient with Beckwith-Wiedemann syndrome: Pediatr Surg Int, 2000; 16(1–2); 124-27
17.. Seki S, Ikenoue T, Murakami N, Ectopic nesidioblastosis: Acta Paediatr Jpn, 1990; 32(3); 308-10
18.. Wild D, Mäcke H, Christ E, Glucagon-like peptide 1 – receptor scans to locate occult insulinomas: N Engl J Med, 2008; 359(7); 766-68
19.. Luo Y, Pan Q, Yao S, Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-exendin-4 for detecting localized insulinoma: A prospective cohort study: J Nucl Med, 2016; 57(5); 715-20
20.. Antwi K, Fani M, Heye T, Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: Evaluation of diagnostic accuracy in a prospective crossover imaging study: Eur J Nucl Med Mol Imaging, 2018; 45(13); 2318-27
21.. Martin-Grace J, O’Tuathail M, Hannon MJ, Amlodipine for the medical treatment of adult-onset diffuse nesidioblastosis: Pancreas, 2015; 44(7); 1162-64
22.. Thompson GB, Andrews JC, Lloyd RV, Noninsulinoma pancreatogenous hypoglycemia syndrome: An update in 10 surgically treated patients: Surgery, 2000; 128(6); 937-45
23.. García-Santos EP, del Carmen Manzanares-Campillo M, Padilla-Valverde D, Nesidioblastosis. A case of hyperplasia of the islets of Langerhans in the adult: Pancreatology, 2013; 13(5); 544-48
24.. De Sousa SM, Haghighi KS, Qiu MR, Synchronous nesidioblastosis, endocrine microadenoma, and intraductal papillary mucinous neoplasia in a man presenting with hyperinsulinemic hypoglycemia: Pancreas, 2016; 45(1); 154-59
25.. Rezvani M, Menias C, Sandrasegaran K, Heterotopic pancreas: histopathologic features, imaging findings, and complications: Radiographics, 2017; 37(2); 484-99
26.. Kim DW, Kim JH, Park SH, Heterotopic pancreas of the jejunum: Associations between CT and pathology features: Abdom Imaging, 2015; 40(1); 38-45
27.. Ma C, Gocke CD, Hruban RH, Belchis DA, Mutational spectrum of intraepithelial neoplasia in pancreatic heterotopia: Hum Pathol, 2016; 48; 117-21
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250