25 June 2020: Articles 
Cirrhosis in a Young Child Due to Fatty Liver; Importance of Early Screening: A Case Report and Review of the Literature
Challenging differential diagnosis, Rare disease
Hamza Hassan Khan1ABCDEF*, Christine E. Klingert12BCDEF, Sanjay Kumar23ADEF, Hernando Lyons23ADOI: 10.12659/AJCR.923250
Am J Case Rep 2020; 21:e923250
Abstract
BACKGROUND: Non-alcoholic fatty liver disease (NAFLD) is the presence of chronic hepatic steatosis in the absence of infections, steatogenic medication use, metabolic/genetic disorders, malnutrition, or ethanol consumption. NAFLD encompasses a spectrum of liver damage varying from non-alcoholic fatty liver (NAFL) on the most clinically benign end of the spectrum to cirrhosis on the opposite extreme, where most liver-related morbidity and mortality occurs.
CASE REPORT: We report a case of a 9-year-old boy with history of obesity (BMI 32.1 kg/m² – 99th percentile) and non-alcoholic fatty liver disease, who was referred to our pediatric gastroenterology clinic with a 1-week history of vomiting and right upper-quadrant abdominal pain. A review of the past medical history revealed transaminitis for the last 4 years and a dietary regimen for the last 2 years with poor compliance and follow-up. An extensive workup revealed an SGPT of 327 unit/L, SGOT 186 unit/L, and triglycerides of 208 mg/dL; infectious, metabolic, genetic, and autoimmune etiologies were ruled-out. The median liver stiffness measured by Fibroscan was 14 kPa, consistent with F4 fibrosis, and the cap median value was 271 dB/mW, reflective of S2 steatosis. An ultrasound-guided core liver biopsy revealed steatohepatitis with bridging and encircling fibrosis consistent with early/evolving cirrhosis.
CONCLUSIONS: Although cirrhosis is rarely seen in pediatric patients with NAFLD, it should always be considered. Secondly, Fibroscan, a non-invasive imaging procedure, is a useful tool to assess the level of fibrosis and steatosis in patients with NAFLD; early evaluation of our patient could potentially have limited the progression to cirrhosis.
Keywords: Fatty Liver, Liver Cirrhosis, Pediatrics, Child, Disease Progression, Early Diagnosis, Non-alcoholic fatty liver disease, Obesity
Background
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver diseases encompassing the accumulation of fatty acids and triglycerides in the liver (hepatocellular macrovesicular steatosis), in the absence of infections, steatogenic medication use, metabolic/genetic disorders, malnutrition, or ethanol consumption [1,2]. NAFLD is typically used to describe a relatively benign condition of steatosis without inflammation or necrosis. This can, however, progress to non-alcoholic steatohepatitis (NASH), which is characterized by inflammation, hepatocyte injury, and cell death [2]. NASH can progress to cirrhosis, a liver disease characterized by scar tissue, leading to liver function impairment, which is a main risk factor for hepatocellular carcinoma [2]. Cirrhosis resulting from NAFLD is uncommon in the pediatric population. Cirrhosis in young pediatric patients is usually a result of genetic abnormalities or biliary atresia [3]. In older children, the leading causes of cirrhosis are autoimmune hepatitis, Wilson’s disease, alpha-1-antitrypsin deficiency, and primary sclerosing cholangitis [3]. The prevalence of NAFLD in the general pediatric population is 3–10% and in obese children it is 23–38% [4,5]. Although NAFLD is commonly recognized in children with obesity, metabolic syndrome, and type 2 diabetes mellitus, there have only been a handful of cases reported in which cirrhosis developed in young children as a result of NAFLD [2]. Progression to cirrhosis is estimated to occur in 6.9–9% of pediatric patients with NAFLD [5,6]. Here, we report a case of a 9-year-old boy with non-alcoholic fatty liver disease that progressed to cirrhosis, initially diagnosed by Fibroscan.
Case Report
A 9-year-old, white, obese boy was referred to our pediatric gastroenterology clinic with vomiting and lower abdominal pain. The symptoms started 1 week prior and included diffuse, crampy abdominal pain with prominence on the right side, intermittent with episodes every 30 minutes that would last for 1–2 minutes and resolved spontaneously. The patient had regular bowel movements with normal consistency and no stool-holding behaviour, dyschezia, or blood or mucus in the stool. The patient had decreased appetite but there was no report of dysphagia, heartburn, chest pain, relief of pain with the passage of a bowel movement, or change in urination. A review of the past medical history revealed transaminitis (initial values: SGPT of 193 unit/L and SGOT of 116 unit/L) for the last 4 years, treated with a dietary intervention (Mediterranean diet), consisting of decreased carbohydrate intake, for the last 2 years. The intervention was ineffective because of poor compliance and follow-up. The family history was significant for maternal history of ischemic heart disease and stage 4 breast cancer, paternal history of polygenic hypercholesterolemia, and the maternal grandfather had colon cancer. There was no family history of obesity, diabetes, or familial hypercholesterolemia.
Because of concerns for appendicitis, the mother took him to the Emergency Department (ED). His height was 1.51 m, weight 73.2 kg, and BMI 32.1 kg/m2 (>99th percentile). The physical examination was unremarkable. An abdominal CT was obtained to rule out appendicitis, revealing decreased liver density compared to the spleen, splenomegaly, and mesenteric lymphadenitis (Figure 1). An ultrasound of the abdomen revealed increased echogenicity of the liver compared to the kidneys (Figure 2). The patient was referred to a pediatric gastroenterologist due to the history of transaminitis and the imaging findings.
In the pediatric gastroenterology clinic, an extensive work-up was performed to evaluate the etiology of transaminitis. Lab findings included an SGPT of 327 unit/L, SGOT 186 unit/L, triglycerides of 208 mg/dL, liver kidney microsomal antibody titers of <1: 20, negative antinuclear antibodies, negative smooth muscle antibody, alpha 1 antitrypsin phenotype M1S, alpha 1 antitrypsin level of 104 mgs/dl, serum ceruloplasmin 35 mgs/dL, negative hepatitis B and C screenings, total IgG of 557 mg/dL, negative lysosomal acid lipase deficiency screening, and prothrombin time of 12.9 s with INR of 0.96. After exclusion of other causes and because of the patient’s history of obesity, a diagnosis of NAFLD was considered. Fibroscan was performed, revealing median liver stiffness of 14 kilopascal (kPa), consistent with stage F4 fibrosis, and the controlled attenuation parameter (CAP) median value was 271 decibels per meter (dB/m), reflective of grade S2 steatosis. An ultrasound-guided core liver biopsy was performed, which showed diffuse, prominent steatosis with associated ballooning degenerative changes of hepatocytes (activity score 2) with bridging and encircling fibrosis consistent with early/evolving cirrhosis (stage 4) (Figure 3).
The patient’s presenting symptoms were initially managed with ondansetron, ketorolac, and hyoscyamine. He was started on vitamin E 800 units/day, and a multi-disciplinary approach was used for management of liver disease by involving a pediatric hepatologist and a nutritional consultation, in addition to the care provided by the pediatric gastroenterologist.
Discussion
The 2 major risk factors for NAFLD are type 2 diabetes and obesity [7], with an emphasis on fat distribution rather than total fat mass [4]. Other risk factors include ethnicity, sex, degree of obesity, insulin resistance, elevated ALT levels, AST/ALT ratio greater than 0.8, hypertension, hypertriglyceridemia, and high IR index [4,7,8]. Studies have shown Hispanic children to be at greater risk of NAFLD and NASH as compared to non-Hispanic children, presenting with higher rates of elevated ALT levels and more advanced fibrosis [4,7]. In adults, women are at increased risk for the development of NAFLD; however, pediatric NAFLD shows a male predominance, with a sex ratio of approximately 2: 1 [7]. Despite the many known factors that contribute to the development of NAFLD, the only variables linked to increased rates of progression to cirrhosis are an elevated ALT level and severe obesity (BMI >95th centile) [7,9].
Hyperinsulinemia has been reported to be the most direct cause of elevated ALT levels in this population [4]. Furthermore, elevated fasting hyperinsulinemia is found in 75% of pediatric patients with NAFLD [4]. Studies have found insulin resistance to be predictive of steatosis, inflammation, and fibrosis [4].
The development of NASH is complex and poorly understood because of the many genetic and environmental factors involved and the lack of long-term studies assessing its pathogenesis [8]. The initial proposed mechanism is a two-hit theory process in which fatty infiltration of the liver resulting from obesity and insulin resistance leads to an increased vulnerability to hepatocyte injury, which is then exacerbated by inflammatory insults from oxidative stress [4,8]. Reactive oxygen species inhibit the mitochondrial respiratory enzymes and cause lipid peroxidation, which ultimately leads to hepatocyte membrane damage [8]. Another, newer, proposed theory involves a multi-hit process involving lipotoxicity, oxidative stress, gut dysbiosis, and endoplasmic reticulum stress pathways [8] and cross-talk between adipose tissue, the pancreas, gut, and liver [9]. Again, the first hit is believed to be a result of obesity and insulin resistance [9]. Liver damage then activates collagen-forming stellate cells, which are responsible for the formation of hepatic fibrosis, and, eventually, cirrhosis [8,9]. Adipose tissue produces many pro-inflammatory cytokines that are involved in the progression of NASH to cirrhosis, including the adipocytokines TNF-alpha, IL-6, leptin, and adiponectin [9]. Genetic factors have also been linked to the development and progression of NAFDL in children [8]. NAFLD shows familial aggregation, being 59% more common in siblings and 78% more common in parents of children with NAFLD [8]. NAFLD is associated with obesity and the PNPLA3 gene [8]. This gene
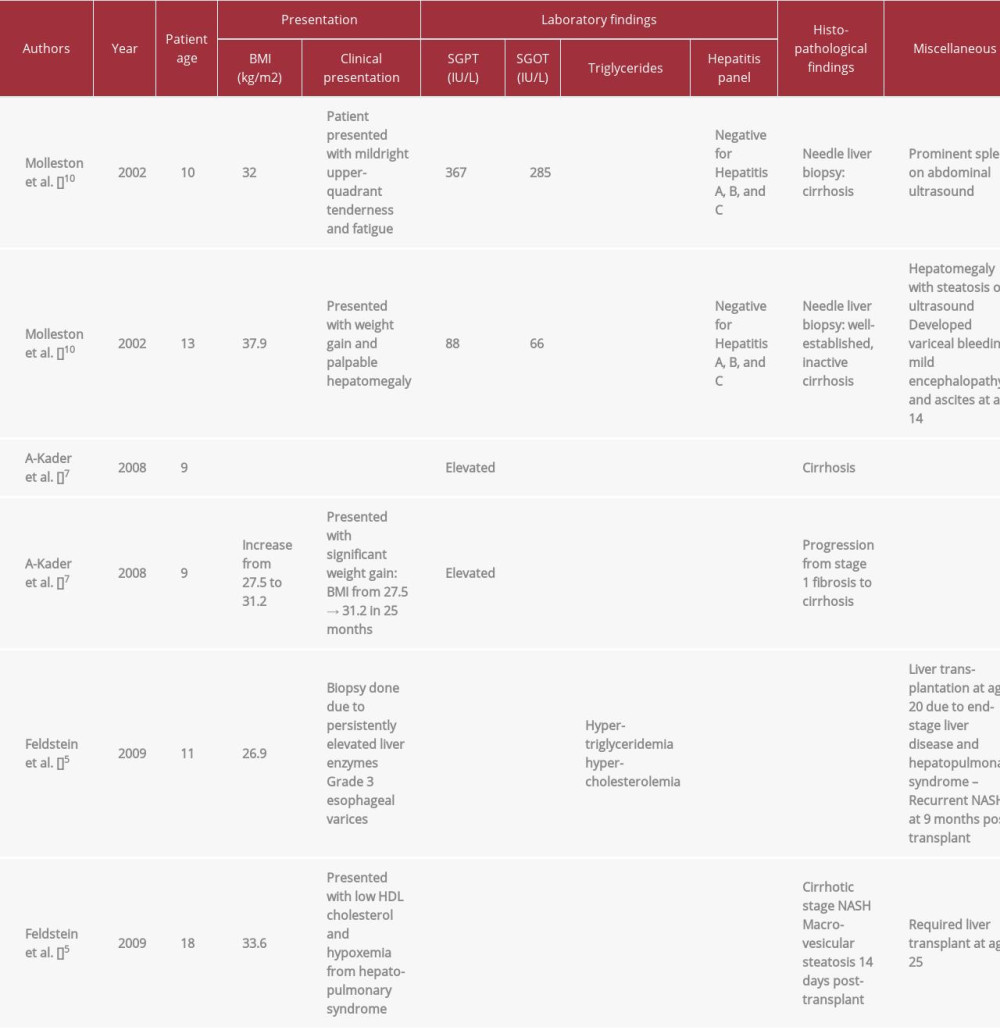
NAFLD is now the most common cause of liver disease in children, mirroring the trend of increasing rates of childhood obesity [2]. To the best of our knowledge, only 6 pediatric patients with cirrhosis secondary to NAFLD have been reported in the literature (Table 1). A-Kader et al. reported 2 nine-year-old children with cirrhosis in their study; similar to our case, one of them had high levels of SGPT and one of them had a high BMI of 31.5 kg/m2 at the time of diagnosis [7]. Both of the patients with liver cirrhosis in Feldstein et al’s study required liver transplantation [5]; one of them was later diagnosed with cirrhosis in the graft accompanied by hepatopulmonary syndrome requiring re-transplantation at 2.3 years after initial transplant [5].
With cirrhosis being an uncommon condition in the pediatric population, it is oftentimes overlooked during the diagnostic workup. Moreover, with the increasing incidence of childhood obesity, we emphasize the importance of early screening, diagnosis, and prompt treatment. Early screening and diagnosis would help catch the disease at an early stage and thus prevent the progression to cirrhosis with appropriate treatment. Furthermore, if not screened early, an increasing number of children would need liver transplantation, burdening the health care economy of the country.
NAFLD has the same diagnostic criteria in children as adults – primarily macrovesicular lipid accumulation present in greater than 5% of hepatocytes [2]. In the pediatric population, 2 forms of NASH exist. NASH 1 is more common in the white population, whereas NASH 2 is more common in males of Asian and Native American race and Hispanic ethnicity. NASH type 1 is the same as adult-type NASH and makes up about 17% of NASH cases in the pediatric population. The diagnostic criteria for NASH type 1 include steatosis, hepatocellular ballooning, and perivenular lobular inflammation [2]. The other form, NASH type 2, has a different set of histologic features unique to children. This form, pediatric NASH, represents 51% of NASH cases in the pediatric population. The histological diagnostic criteria for NASH type 2 include portal-based chronic inflammation and fibrosis, moderate to severe steatosis, and the absence of zone 3 lesions [2]. Pediatric patients with NASH may possess features of both NASH types 1 and 2 [2]. Both NASH types 1 and 2 can progress to fibrosis and then cirrhosis [2]. Our patient had NASH 1.
The traditional criterion standard for diagnosing cirrhosis has been liver biopsy, which is an invasive technique and is thus associated with significant risks of morbidity, mortality, and healthcare and economic burden. We utilized transient elastography (Fibroscan) in this patient’s evaluation.
Fibroscan is based upon ultrasound technology that can measure the degree of steatosis and fibrosis of the liver [11]. Steatosis is measured in decibels/meter (dB/m) and ranges from 100 to 400 dB/m and is graded from S1 to S3. The grading is done as follows: 238–260 dB/m is graded S1, which is reflective of 11–33% fatty liver; S2 is graded 260–290 dB/m, which is reflective of 34–66% fatty liver, and values higher than 290 dB/m are graded S3, which correlates with 67% or more of fatty liver [11]. Our patient had grade 2 steatosis with a CAP median value of 271 dB/m.
The degree of fibrosis is measured in kilopascals (kPa) and normally ranges from 2–14 kPa. Fibrosis is graded F0–F4: F0 and F1 (no or mild liver scarring) are 2–7 kPa, F2 (moderate liver scarring) is 7.5–10 kPa, F3 (severe liver scarring) is 10–14 kPa, and F4 includes values of 14 kPa and above, which indicates advanced liver scarring (cirrhosis) [11]. Our patient had grade 4 fibrosis with a value of 14 kPa. According to the American Association for the Study of Liver Disease (AASLD), patients with grade F3 and F4 should undergo a liver biopsy; hence, our patient underwent an ultrasound-guided core liver biopsy.
Fibroscan is quick and non-invasive, making it a great tool for cirrhosis screening. Elzawawy et al. found Fibroscan to have high sensitivity and specificity for diagnosing cirrhosis [12]. As Fibroscan use becomes more widespread, the workup of cirrhosis in adults and children alike should become more reliable and less invasive, leading to quicker diagnosis and treatment.
The ultrasound-guided core liver biopsy revealed a NAFLD activity score of 2 and stage 4 NASH. Brunt et al. classified NASH histopathologically into 4 stages based upon the location and the degree of fibrosis: stage 1 is zone 3 perisinusoidal fibrosis, stage 2 is portal fibrosis in the presence of stage 1, stage 3 is bridging fibrosis in addition to stage 2, and stage 4 is cirrhosis [13,14]. The NASH Clinical Research Network (NASH CRN) designed an activity score for the full spectrum of NAFLD; steatosis is scored 0–3, with a score of 0 for <5% steatosis, a score of 1 for 5–33% steatosis, a score of 2 for >33–66% steatosis, and a score of 3 for steatosis >66% [14,15].
Conclusions
This case report demonstrates the rapid progression to cirrhosis in a young pediatric patient. The incidence of childhood obesity is on the rise, leading to growing numbers of children with NAFLD. With increasing liver disease, physicians need to be more aware of the potential for development of cirrhosis at younger ages. Physicians should also be aware of the utility of Fibroscan in the diagnostic workup of cirrhosis in pediatric patients to ensure prompt diagnosis and appropriate treatment.
Figures
References:
1.. Vos MB, Abrams SH, Barlow SE, NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN): J Pediatr Gastroenterol Nutr, 2017; 64(2); 319-34
2.. Fleet SE, Lefkowitch JH, Lavine JE, Current concepts in pediatric nonalcoholic fatty liver disease: Gastroenterology Clin North Am, 2017; 46(2); 217-31
3.. Pinto RB, Schneider AC, da Silveira TR, Cirrhosis in children and adolescents: An overview: World J Hepatol, 2015; 7(3); 392-405
4.. Patton HM, Sirlin C, Behling C, Pediatric nonalcoholic fatty liver disease: A critical appraisal of current data and implications for future research: J Pediatr Gastroenterol Nutr, 2006; 43(4); 413-27
5.. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, the natural history of nonalcoholic fatty liver disease in children: A follow-up study for up to 20-years: Gut, 2009; 58(11); 1538-44
6.. Kohli R, Boyd T, Lake K, Rapid progression of NASH in childhood: J Pediatr Gastroenterol Nutr, 2011; 50(4); 453-56
7.. A-Kader HH, Henderson J, Vanhoesen K, Nonalcoholic fatty liver disease in children: A single-center experience: Clin Gastroenterol Hepatol, 2008; 6(7); 799-802
8.. Bush H, Golabi P, Younossi ZM, Pediatric non-alcoholic fatty liver disease: Children (Basel), 2017; 4(6); E48
9.. Temple JL, Cordero P, Li J, A Guide to non-alcoholic fatty liver disease in childhood and adolescence: Int J Mol Sci, 2016; 17(6); E947
10.. Molleston JP, White F, Teckman J, Fitzgerald JF, Obese children with steatohepatitis can develop cirrhosis in childhood: Am J Gastroenterol, 2002; 97(9); 2460-62
11.. , Understanding your FibroScan® Results: Memorial Sloan Kettering Cancer Center, 2018 https://www.mskcc.org/cancer-care/patient-education/understanding-your-fibroscan-results
12.. Elzawawy MS, Hassanein SA, Nomrosy RME, The role of fibroscan in assessment of liver cirrhosis in patients with chronic liver disease: Menoufia Medical Journal, 2018; 31(2); 520-24
13.. Brunt EM, Janney CG, Di Bisceglie AM, Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions: Am J Gastroenterol, 1999; 94(9); 2467-74
14.. Takahashi Y, Fukusato T, Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: World J Gastroenterol, 2014; 20(42); 15539-48
15.. Kleiner DE, Brunt EM, Van Natta M, Design and validation of a histological scoring system for nonalcoholic fatty liver disease: Hepatology, 2005; 41(6); 1313-21
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250