15 August 2020: Articles 
A 57-Year-Old African American Man with Severe COVID-19 Pneumonia Who Responded to Supportive Photobiomodulation Therapy (PBMT): First Use of PBMT in COVID-19
Unusual or unexpected effect of treatment
Scott A. Sigman1ABCDEF*, Soheila Mokmeli2ABCDEF, Monica Monici3A, Mariana A. Vetrici4BCDEFDOI: 10.12659/AJCR.926779
Am J Case Rep 2020; 21:e926779
Abstract
BACKGROUND: Coronavirus disease 2019 (COVID-19) is associated with lung inflammation and cytokine storm. Photobiomodulation therapy (PBMT) is a safe, non-invasive therapy with significant anti-inflammatory effects. Adjunct PBMT has been employed in treating patients with lung conditions. Human studies and experimental models of respiratory disease suggest PBMT reduces inflammation and promotes lung healing. This is the first time supportive PBMT was used in a severe case of COVID-19 pneumonia.
CASE REPORT: A 57-year-old African American man with severe COVID-19 received 4 once-daily PBMT sessions by a laser scanner with pulsed 808 nm and super-pulsed 905 nm modes for 28 min. The patient was evaluated before and after treatment via radiological assessment of lung edema (RALE) by CXR, pulmonary severity indices, blood tests, oxygen requirements, and patient questionnaires. Oxygen saturation (SpO₂) increased from 93–94% to 97–100%, while the oxygen requirement decreased from 2–4 L/min to 1 L/min. The RALE score improved from 8 to 5. The Pneumonia Severity Index improved from Class V (142) to Class II (67). Additional pulmonary indices (Brescia-COVID and SMART-COP) both decreased from 4 to 0. CRP normalized from 15.1 to 1.23. The patient reported substantial improvement in the Community-Acquired Pneumonia assessment tool.
CONCLUSIONS: This report has presented supportive PBMT in a patient with severe COVID-19 pneumonia. Respiratory indices, radiological findings, oxygen requirements, and patient outcomes improved over several days and without need for a ventilator. Future controlled clinical trials are required to evaluate the effects of PBMT on clinical outcomes in patients with COVID-19 pneumonia.
Keywords: Anti-Inflammatory Agents, COVID-19, Laser Therapy, Respiratory Distress Syndrome, Adult, African Americans, Betacoronavirus, COVID-19, Coronavirus Infections, Low-Level Light Therapy, Pandemics, Pneumonia, Viral, SARS-CoV-2, Tomography, X-Ray Computed, United States
Background
Coronavirus disease 2019 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The presentation of COVID-19 includes dyspnea, lung edema, and pneumonia. Morbidity and mortality are associated with Acute Respiratory Distress Syndrome (ARDS) and cytokine storm. Hospitalized COVID-19 patients are classified as severe if they require intensive care unit (ICU) admission [1,2]. Here, we report the first case of the use of supportive or adjunctive photobiomodulation therapy (PBMT) in a patient with severe COVID-19 pneumonia.
PBMT is an emerging alternative modality with demonstrated anti-inflammatory effects in pain management, lymphedema, wound healing, and musculoskeletal injuries. Additional terms for PBMT include low-level laser (or light) therapy (LLLT), cold laser, and photobiostimulation [3]. The effects of PBMT differ from the thermal effects produced by the high-power lasers used in cosmetic and surgical procedures to destroy the tissue [4,5]. PBMT utilizes non-ionizing, non-thermal light sources in the visible and infrared spectra (400–1000 nm) [3]. In PBMT, light is applied over damaged tissues and the light energy absorbed by intracellular chromophores or biomolecules starts a cascade of molecular reactions that improve cell function and enhance the body’s healing process [4]. In effect, light stimulates healing, modulates the immune system, and reduces inflammation, edema, and pain [4]. PBMT is non-invasive, cost-effective, and has no known adverse effects.
Empirical use of PBMT in children, adults, and elderly patients with pneumonia, asthma, chronic bronchitis, or pulmonary fibrosis resulted in reduced chest pain and heaviness, normalization of respiratory function, shortened recovery times, and improved immunological and radiological parameters. In these patients, PBMT used in combination with conventional medical treatment was safe and appeared to produce a synergistic effect in healing [6–10]. Recent publications recommend the use of supportive PBMT in COVID-19 patients [11–13]. ARDS is a critical complication of COVID-19 infection and supportive PBMT can ameliorate ARDS and promote lung healing [11,13–18]. Animal models of acute inflammation of the respiratory system suggest that transcutaneous PBMT over the lungs is effective against cytokine storm and ARDS via its anti-inflammatory action at multiple levels [14–18].
The theory of supportive PBMT for COVID-19 is based on laser light reaching lung tissue, which relieves inflammation and promotes healing. The World Association for Laser Therapy recommended treatment doses for low-level laser therapy, or PBMT for superficial to deep tissue lesions in musculoskeletal disorders in 2010 [19].
The minimum observed therapeutic dose for a bio-stimulatory effect of red and near-infrared (NIR) lasers is 0.01 J/cm2 [20]. NIR Laser light at a power of 1 W/cm2 projected through bovine tissue ranging in thickness from 1.8 to 9.5 cm resulted in effective power densities at 3.4 cm and 6.0 cm [21]. In veterinary practice, feline and canine pneumonia is frequently treated with laser doses of 6–10 J/cm2 [22]. These animals have a thicker chest wall and furry skin, making penetration more challenging than in humans. Therefore, the range used in cats and dogs approximates an effective dose for humans.
Our previous experience in treating asthma [23] and musculo-skeletal pain and injuries suggested that the anti-inflammatory effects of PBMT could benefit the severe inflammatory condition in COVID-19 patients. The laser machine used in this case is an US Food and Drug Administration (FDA)-cleared system for pain management and inflammation reduction in deep joints of the body. The combination of 808 and 905 nm, both NIR wavelengths, provides penetration to depths of 4–5.4 cm. This laser machine is used for deeper tissues like hips and pelvic joints that are surrounded by thick muscles. The therapeutic dose with this machine is 4.5 J/cm2 over the skin to reach these deep targets of the pelvis. Based on our calculations, we used 7.2 J/cm2 over the skin to deliver just over 0.01 J/cm2 of laser energy to the lung. The 7.2 J/cm2 dosage penetrates the chest wall (1.6 to 6 cm in humans) and reaches the lung tissue with sufficient energy for bio-stimulation. Scapular protraction in the prone position reduces the bone and muscle tissue the laser must penetrate, thereby increasing laser energy to the lung fields.
Here, we report the first use of PBMT as a supportive treatment in a severe case of COVID-19 pneumonia.
Case Report
A 57-year-old African American man with a history of hypertension and asthma presented with shortness of breath, severe dehydration, acute renal failure, and
The patient was treated with an FDA-cleared Multiwave Locked System (MLS) Therapy Laser (ASA Laser, Italy.) The MLS laser utilizes a mobile scanner with 2 synchronized laser diodes, one in pulse mode (adjustable to 1–2000 Hz), emitting at 905 nm, and another in pulsed mode emitting at 808 nm. The 2 laser beams work simultaneously and synchronously. This laser is used in pain centers for treatment of musculoskeletal pain and inflammation. Laser parameters were set as outlined in Table 1 and Figure 1. The laser scanner was adjusted to 20 cm above the skin, as recommended by the manufacturer. Each lung was scanned for 14 min from apex to base over 250 cm2 of the posterior thorax (Table 1, Figures 1, 2).
Specific prone positioning was used with the patient’s hands under his head for maximal scapular protraction. The laser field was focused to the medial border of the scapula opening the lung fields, thereby minimizing the chest wall thickness for theoretical improvement of laser penetration to lung tissue.
Prior to laser treatment, the patient was bedridden, with SpO2 92–95% on 2–4 L/min oxygen. He had completed his antibiotic course and was not receiving any pharmacotherapeutic or IV support. He experienced severe paroxysmal coughing episodes and had failed a physical therapy trial. The patient tolerated the prone position for laser treatment for a total of 28 min. Within 5 min of laser treatment, his oxygen saturation rose from 94% to 100% in the first session. Following treatment, he returned to his bed and resumed the semi-sitting position and SpO2 remained at 98% for the rest of the day.
The patient tolerated all 4 daily treatments and noted significant improvement in breathing immediately after each treatment. Paroxysmal coughing spells resolved after the third treatment. Upon completion of the fourth treatment, the patient was able to ambulate in the room with physical therapy. On the day following his final treatment, the patient was discharged to an acute rehabilitation facility on 1 L/min oxygen. On the day after arrival to the acute rehabilitation facility, the patient was able to complete 2 trials of stair climbing with physical therapy and was in the process of weaning to room air.
The patient’s response to PBMT was evaluated by comparing different scoring tools before and after laser therapy. The patient showed improvement in all evaluation criteria (Table 2).
The Pneumonia Severity Index (PSI) [24] calculates the probability of morbidity and mortality among patients with community-acquired pneumonia (CAP). Prior to treatment, the patient’s PSI score was Class V (142), which requires ICU treatment and predicts intubation and ventilator use. After PBMT, PSI decreased to Class II (67), which signifies outpatient treatment.
The SMART-COP score [25], which is an acronym for Systolic blood pressure, Multilobar infiltrates, Albumin, Respiratory rate, Tachycardia, Confusion, Oxygen, and pH, evaluates pneumonia severity and predicts the need for intensive respiratory or vasopressor support (IRVS) in CAP. The pretreatment SMART-COP score was 5, placing him in the high-risk group, and signifying a 1 in 3 chance of needing IRVS. Following PBMT, the SMART-COP decreased to 2, implying minimal risk for needing IRVS.
The Brescia-COVID Respiratory Severity Scale [26] is a stepwise algorithm for managing patients with confirmed COVID-19. Before treatment, the patient’s score was 4 out of 4, which requires a trial of high-flow nasal cannula (HFNC), reassessment, and intubation if the score remains >2. Following PBMT, the patient’s Brescia-COVID score was 0, which simply requires patient monitoring.
The CAP tool score [27] is a short and sensitive questionnaire evaluating changes in respiratory symptoms and well-being during the treatment of community-acquired pneumonia. Scores <75% indicate symptomatic distress. The patient’s pretreatment CAP score was 36.68% and increased to 82.84% after treatment. His CAP Respiratory Score improved from 67.52%, before treatment to 87.17% at the time of discharge. The CAP Well-Being score increased from 0% before treatment to 73.07% after treatment. This patient demonstrated substantial improvement in all 3 measures of respiratory symptoms.
The Radiographic Assessment of Lung Edema (RALE) score [28,29] evaluates lung edema on CXR in ARDS patients. To quantify the extent of infection, a severity score was calculated [29]. A score of 0 to 4 was assigned to each lung depending on the percent lung consolidation or ground-glass opacity, with 0 signifying no lung involvement, 1 indicating <25% lung involvement, 2 indicating 25–50% lung involvement, 3 indicating 50–75% lung involvement, and 4 indicating >75% involvement. The scores for each lung were added together to produce the final severity score [29]. The RALE score was 8 (>75% involvement of both lungs) and improved to 5 upon treatment completion (Figure 3).
His white blood cell count decreased from 10.7 to 6.5 and his C-reactive protein decreased from 15.1 to 1.23 after treatment. The oxygen requirement before treatment was 2–4 L/min with an oxygen saturation (SpO2) of 93–94%. The oxygen requirement after treatment improved to 1 L/min with an SpO2 of 97–100% at the time of discharge.
Discussion
This case report showed that 4 daily sessions of adjunct PBMT were beneficial in a patient with severe COVID-19 symptoms. The patient’s positive response to treatment was supported by radiological findings, pulmonary severity scores, oxygen requirements, blood and inflammatory markers, and patient questionnaires. On follow-up, his clinical recovery in total was 3 weeks, whereas the median time for COVID-19 is typically 6–8 weeks [30].
The therapeutic effects of PBMT on pneumonia are thought to occur via local and systemic effects that reduce inflammatory cytokines, cellular infiltrates, edema and fibrosis, and increase anti-inflammatory cytokines and processes, and promote healing. Local PBMT affects the entire body when photoproducts are distributed via the vasculature to reach distant targets. Activated photoproducts lead to alleviation of inflammation and immunomodulatory effects, and stimulate wound healing and tissue regeneration [4]. Animal studies illustrate the potency of PBMT.
Transcutaneous PBMT in murine models for pulmonary fibrosis and ARDS significantly reduced pro-inflammatory cytokines, inflammatory cells, and collagen fiber deposition in lung parenchyma [14–18]. In contrast, the anti-inflammatory cytokine interleukin-10, serum monocytes, and lung macrophages were significantly increased following PBMT [15,17]. The molecular basis of MLS laser anti-inflammatory effects has been demonstrated in murine and
Human trials have shown local and systemic effects of PBMT when applied to quadriceps muscle in patients with chronic obstructive pulmonary disease [10]. Beneficial effects extended beyond improved muscular performance, to statistically significant reductions in dyspnea and fatigue [10]. Our patient also reported subjective feelings of improved respiratory function and strength.
Our patient was only placed in the prone position for the duration of laser treatment. Treatments lasted exactly 28 min for each of the 4 days. Physiological evidence and clinical trial data support the use of prone position ventilation in selected patients with moderate-to-severe ARDS. For patients to benefit, the use of long prone positioning sessions of 12 h to 18 h per session are necessary [34,35]. An increase in SpO2 from 94% to 100% occurred within the first 5 min of treatment, and the patient maintained good saturation thereafter. This finding shows the rapid effect of PBMT treatment on oxygen saturation. It is unlikely that prone positioning alone was the reason for improved oxygenation, given the minimal time in that position.
A strength of this case report is that we collected patient symptom data before and after treatment. All 4 pulmonary scoring tools and the 3 patient questionnaires demonstrated the benefit of treatment. To the best of our knowledge, this was the first time that PBMT was used as adjunctive treatment for pneumonia in a COVID-19 patient. Irradiation over the posterior projection of the lungs, using the scanning method, has no risk of contamination since the scanning laser does not physically touch the patient. A deficiency of our study is the lack of inflammatory markers and blood tests. Future studies should include measurements before and after treatment of interleukin-6, interleukin-10, TNF-α, as well as additional inflammatory markers. A limitation of this case report is that this is a single patient and we were unable to carry out any statistical analysis.
Conclusions
This report has presented a patient with severe COVID-19 pneumonia associated with ARDS who was given supportive treatment with PBMT. Based on this case report, as well as clinical experience of PBMT in respiratory tract diseases in humans, we consider PBMT to be a feasible adjunct modality for the treatment of COVID-19. There is published experimental work demonstrating the anti-inflammatory effect of PBMT on lung tissue. We suggest that the use of adjunct PBMT in the early stages of severe ARDS seen in COVID-19 patients can enhance healing and reduce the need for prolonged ventilator support and ICU stay. The urgent current medical situation calls for PMBT pilot studies and clinical trials to evaluate its effect on COVID-19 pneumonia. This patient is part of an ongoing investigational randomized controlled trial.
Figures
References:
1.. Liang T: Handbook of COVID-19 prevention and treatment March, 2020, Zhejiang University School of Medicine
2.. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;]: Lancet, 2020; 395(10223); 497-506
3.. Anders JJ, Lanzafame RJ, Arany PR, Low-level light/laser therapy versus photobiomodulation therapy: Photomed Laser Surg, 2015; 33(4); 183-84
4.. Cotler HB, Chow RT, Hamblin MR, Carroll J, The use of low-level laser therapy (LLLT) for musculoskeletal pain: MOJ Orthop Rheumatol, 2015; 2(5); 00068
5.. Hamblin MR, Mechanisms and applications of the anti-inflammatory effects of photobiomodulation: AIMS Biophys, 2017; 4(3); 337-61
6.. Amirov NB, [Parameters of membrane permeability, microcirculation, external respiration, and trace element levels in the drug-laser treatment of pneumonia]: Ter Arkh, 2002; 74(3); 40-43 [in Russsian]
7.. Derbenev VA, Mikhailov VA, Denisov IN, Use of low-level laser therapy (LLLT) in the treatment of some pulmonary diseases:: Ten-year experience Proceedings of the SPIE Oct 28–31, 1999; 4166; 323-25, Florence, Italy, SPIE digital library 2000
8.. Ostronosova NS, [Outpatient use of laser therapy in bronchial asthma]: Ter Arkh, 2006; 78(3); 41-44 [in Russsian]
9.. Mehani SHM, Immunomodulatory effects of two different physical therapy modalities in patients with chronic obstructive pulmonary disease: J Phys Ther Sci, 2017; 29(9); 1527-33
10.. Miranda EF, de Oliveira LV, Antonialli FC, Phototherapy with combination of super-pulsed laser and light-emitting diodes is beneficial in improvement of muscular performance (strength and muscular endurance), dyspnea, and fatigue sensation in patients with chronic obstructive pulmonary disease: Lasers Med Sci, 2015; 30(1); 437-43
11.. Enwemeka CS, Bumah VV, Masson-Meyers DS, Light as a potential treatment for pandemic coronavirus infections: A perspective: J Photochem Photobiol B, 2020; 207; 111891
12.. Fekrazad R, Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 management: Photobiomodul Photomed Laser Surg, 2020; 38(5); 255-57
13.. Mokmeli S, Vetrici M, Low-level laser therapy as a modality to attenuate cytokine storm at multiple levels, enhance recovery, and reduce the use of ventilators in COVID-19: Can J Respir Ther, 2020; 56; 1-7
14.. Aimbire F, Lopes-Martins RA, Albertini R, Effect of low-level laser therapy on hemorrhagic lesions induced by immune complex in rat lungs: Photomed Laser Surg, 2007; 25(2); 112-17
15.. de Brito AA, da Silveira EC, Rigonato-Oliveira NC, Low-level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis: Relevance to cytokines secretion from lung structural cells: J Photochem Photobiol B, 2020; 203; 111731
16.. Cury V, de Lima TM, Prado CM, Low-level laser therapy reduces acute lung inflammation without impairing lung function: J Biophotonics, 2016; 9(11–12); 1199-207
17.. da Cunha Moraes G, Vitoretti LB, de Brito AA, Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor: Oxid Med Cell Longev, 2018; 2018; 6798238
18.. Miranda da Silva C, Peres Leal M, Brochetti RA, Low-level laser therapy reduces the development of lung inflammation induced by formalde-hyde exposure: PLoS One, 2015; 10(11); e0142816
19.. , Dosage recommendations. Recommended treatment doses for low-level laser therapy Available at URL: ; https://waltza.co.za/documentation-links/recommendations/dosage-recommendations/
20.. Tunér J, Hode L, Laser therapy, clinical practice and scientific background: Grängesberg, 2002, Sweden, Prima Books AB
21.. Hudson DE, Hudson DO, Wininger JM, Richardson BD, Penetration of laser light at 808 and 980 nm in bovine tissue samples: Photomed Laser Surg, 2013; 31(4); 163-68
22.. Arza RA, Upper and lower respiratory conditions: Laser therapy in veterinary medicine, 2017; 150-60, Hoboken, John Wiley & Sons, Inc
23.. Vatankhah Z, Mokmeli S, Boshbishe S, Evaluation of the effect of low-level laser therapy (LLLT) in the treatment of asthma, added to conventional drug therapy (crossover, case control clinical trial): Photodiagnosis and Photodynamic Therapy, 2008; 5(Suppl. 1); S22
24.. , Community-Acquired Pneumonia Severity Index (PSI) for Adults Calculator https://www.merckmanuals.com/medical-calculators/CommunityAcqPneumonia.htm
25.. Charles PG, Wolfe R, Whitby M, SMART-COP: A tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia: Clin Infect Dis, 2008; 47(3); 375-84
26.. Duca A, Piva S, Focà E, Calculated decisions: Brescia-COVID Respiratory Severity Scale (BCRSS)/algorithm: Emerg Med Pract, 2020; 22(5 Suppl.); CD1–2
27.. El Moussaoui R, Opmeer BC, Bossuyt PM, Development and validation of a short questionnaire in community acquired pneumonia: Thorax, 2004; 59(7); 591-95
28.. Zimatore C, Pisani L, Lippolis V, The radiographic assessment of lung edema (RALE) score has excellent diagnostic accuracy for ARDS: Eur Respir J, 2019; 54(Suppl. 63); OA3299
29.. Wong HYF, Lam HYS, Fong AH, Frequency and distribution of chest radiographic findings in patients positive for COVID-19: Radiology, 2020; 296(2); E72-78
30.. Phua J, Weng L, Ling L, Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations [published correction appears in Lancet Respir Med. 2020 May;8(5): e42]: Lancet Respir Med, 2020; 8(5); 506-17
31.. Micheli L, Cialdai F, Pacini A: Sci Rep, 2019; 9(1); 9297
32.. Micheli L, Di Cesare Mannelli L, Lucarini E, Photobiomodulation therapy by NIR laser in persistent pain: An analytical study in the rat: Lasers Med Sci, 2017; 32(8); 1835-46
33.. Monici M, Cialdai F, Ranaldi F, Effect of IR laser on myoblasts: A proteomic study: Mol Biosyst, 2013; 9(6); 1147-61
34.. Henderson WR, Griesdale DE, Dominelli P, Ronco JJ, Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome?: Can Respir J, 2014; 21(4); 213-15
35.. Guérin C, Reignier J, Richard JC, Prone positioning in severe acute respiratory distress syndrome: N Engl J Med, 2013; 368(23); 2159-68
Figures
Tables
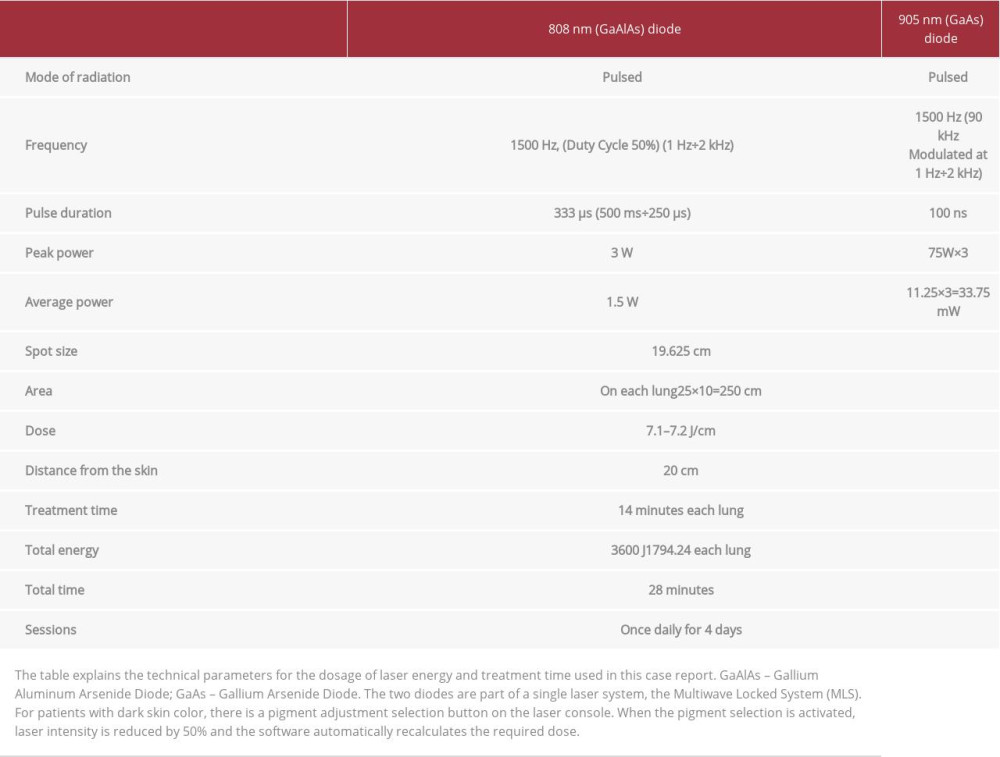 Table 1.. Laser parameters for COVID-19 pneumonia patients.
Table 1.. Laser parameters for COVID-19 pneumonia patients.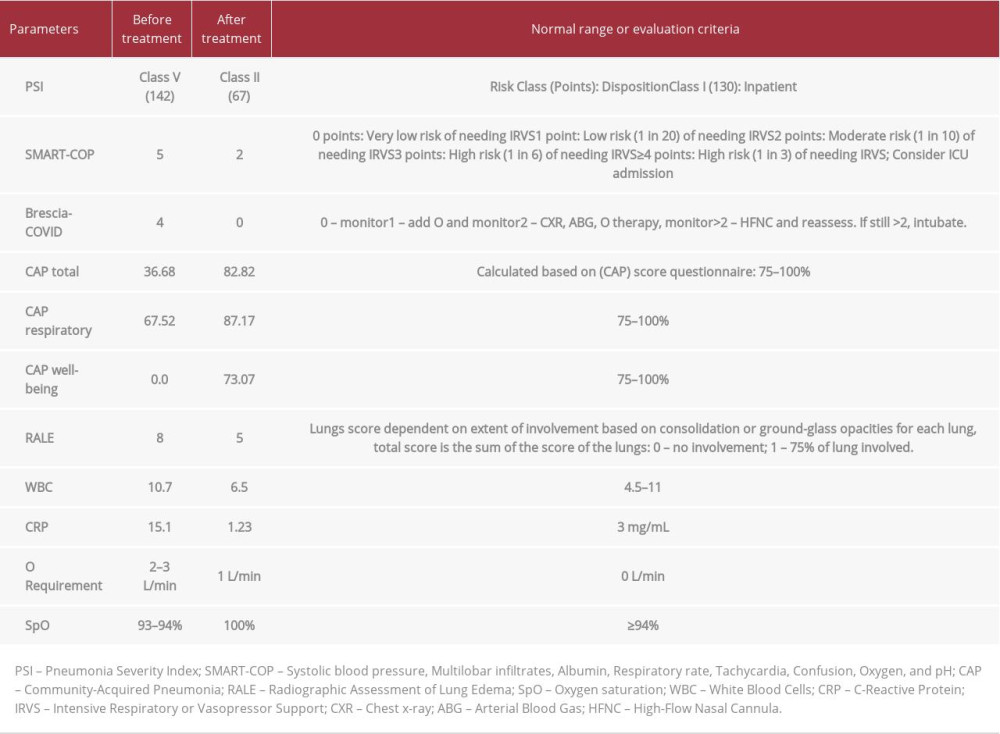 Table 2.. Evaluation criteria before and after photobiomodulation therapy in a COVID-19 patient.
Table 2.. Evaluation criteria before and after photobiomodulation therapy in a COVID-19 patient. Table 1.. Laser parameters for COVID-19 pneumonia patients.
Table 1.. Laser parameters for COVID-19 pneumonia patients. Table 2.. Evaluation criteria before and after photobiomodulation therapy in a COVID-19 patient.
Table 2.. Evaluation criteria before and after photobiomodulation therapy in a COVID-19 patient. In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








