15 March 2021: Articles 
Florid Interstitial Hemorrhages: A Novel Feature of Amoxicillin-Clavulanate-Induced Acute Tubulointerstitial Nephritis
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Adverse events of drug therapy
Muhammad Asim1ABCDEF*, Farooq Ahmad1BCDE, Mohammed Akhtar2BCDEFDOI: 10.12659/AJCR.928989
Am J Case Rep 2021; 22:e928989
Abstract
BACKGROUND: Acute tubulointerstitial nephritis is most often induced by drug therapy and is characterized by the presence of edema, inflammatory infiltrates, and sometimes granulomas within the interstitium. We report this case to describe florid interstitial hemorrhages as a novel feature of Amoxicillin-Clavulanate-induced acute tubulointerstitial nephritis.
CASE REPORT: A young man presented with intermittent visible hematuria and acute kidney injury after a course of Amoxicillin-Clavulanate for upper respiratory tract illness. Renal biopsy demonstrated acute tubulointerstitial nephritis with multifocal intense interstitial hemorrhages, intratubular red blood cells, and red blood cell casts. At the same time, he was diagnosed with acute lymphoblastic leukemia. Leukemic cellular infiltration and other potential causes of tubulointerstitial nephritis were ruled out.
CONCLUSIONS: Drug-induced tubulointerstitial nephritis can be associated with florid interstitial hemorrhages. This can lead to an atypical clinicopathological presentation of tubulointerstitial nephritis, masquerading as glomerulonephritis, vasculitis, or infectious interstitial nephritis.
Keywords: Amoxicillin, Hematuria, Hemorrhage, Nephritis, Interstitial, Clavulanic Acid, Glomerulonephritis
Background
Over two-thirds of tubulointerstitial nephritis (TIN) cases are drug-induced, most commonly due to non-steroidal anti-inflammatory agents and antibiotics. Beta-lactam antibiotics are among the worst precipitating agents, causing tubulointerstitial nephritis in days to weeks following exposure via cell-mediated immunity [1].
The histopathological hallmark of drug-induced TIN is inter-stitial edema associated with inflammatory cells, within the renal interstitium, whereas the glomeruli and blood vessels are spared [2]. Interstitial infiltrates are mostly composed of lymphocytes, macrophages, eosinophils and plasma cells. Sometimes, interstitial granulomas may be observed. The usual renal presentation is with acute/subacute kidney injury associated with pyuria, white blood cell casts, microscopic hematuria, and low-grade proteinuria [3].
We report this case to describe florid interstitial hemorrhages as a novel feature of Amoxicillin-Clavulanate-induced TIN. This was associated with visible hematuria and red blood cell casts, which are features uncharacteristic of TIN.
Case Report
A 33-year-old Arab male patient was admitted via the emergency department with intermittent painless visible hematuria of 3 weeks’ duration. Three weeks before the onset of hematuria, he had a throat infection for which he took a 2-week course of Amoxicillin-Clavulanate. He denied any previous episodes of hematuria, family history of renal disease, intake of non-steroidal anti-inflammatory agents or any medication other than Amoxicillin-Clavulanate. One week before admission, he had consulted a physician for bilateral flank pain. At that time, laboratory tests revealed a serum creatinine of 106 μmol/L, microscopic hematuria, and pyuria. The coagulation profile was normal. A non-contrast computed tomography scan ruled out nephrolithiasis, pyelonephritis, and urinary tract obstruction.
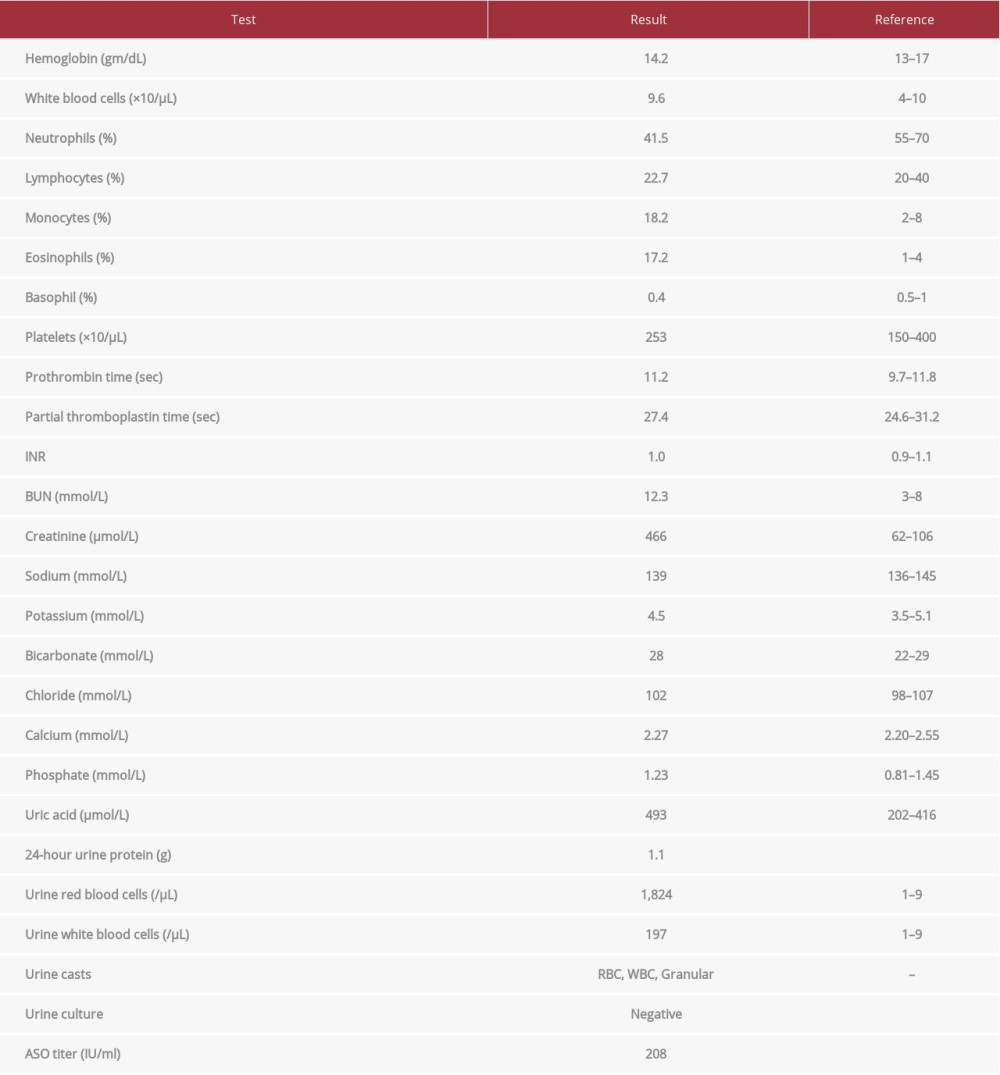
At the time of admission, the patient was apyrexial. Blood pressure was 127/79 mmHg. There was no evidence of skin rash, arthritis, or facial/peripheral edema. Posterior cervical, supraclavicular, and axillary lymphadenopathy was noted. Lymph nodes were 2-3 cm in size, non-tender, and mobile. The rest of the physical examination was unremarkable. Basic laboratory studies (summarized in Table 1) disclosed severe renal dys-function, with serum creatinine of 466 μmol/L. This was associated with hematuria, pyuria, and low-grade proteinuria, but no bacteriuria or crystaluria. A Doppler ultrasound scan of the kidneys was normal. Renal biopsy was organized to exclude rapidly progressive glomerulonephritis and tubulointerstitial nephritis. A peripheral blood smear demonstrated eosinophilia, monocytosis, and circulating blast cells, prompting urgent bone marrow examination. An immunology workup was negative for antinuclear, anti-glomerular basement membrane, and anti-neutrophil cytoplasmic antibodies. Serum C3 and C4 levels were normal. Serology was negative for HBV, HCV, and HIV, and serum protein electrophoresis did not show any monoclonal bands.
A renal biopsy was performed on day 2 of admission. All salient histopathological findings were limited to the interstitial compartment and tubules. The cortical and medullary interstitium was edematous and infiltrated with a mixture of lymphocytes, plasma cells, and eosinophils (Figure 1). However, the most striking feature was florid multifocal interstitial hemorrhages (Figure 2). Abundant red blood cells in several tubules and red blood cell casts in some tubules were also seen. The glomeruli appeared normal, with patent capillary lumina and normal thickness of the capillary walls. No red blood cells were noted in the Bowman’s spaces. Direct immunofluorescence studies did not show any significant deposition of immunoglobulins (IgG, IgA, and IgM), complement components (C3, C1q), fibrinogen, or kappa/lambda light chains. Small arteries and arterioles were within normal limits. Mild (5-10%) focal tubular atrophy and interstitial fibrosis was seen. Hence, a diagnosis of drug-induced acute TIN was made. Bone marrow aspiration and trephine biopsy was done on day 3 of admission; flow cytometry confirmed the diagnosis of T cell acute lymphoblastic leukemia. The diagnosis of acute lymphoblastic leukemia broadened the differential diagnosis of renal dysfunction, as kidney injury from leukemia or its complications is well recognized. Immunohistochemical staining was carried out by an experienced hematopathologist to detect possible leukemic infiltration; staining was negative for TdT, CD10, CD7, CD99, and CD56. SV40 antibody staining for BK virus was also negative.
The patient was transferred to the Hematology/Oncology Center. Treatment of T cell acute lymphoblastic leukemia was initiated according to the UKALL 14 protocol on day 6 of admission. At that time, serum creatinine was 822 μmol/L, although the patient remained non-oliguric with no significant electrolyte or acid-base disturbances. Dexamethasone was given at a dose of 16 mg/day for 7 days (Steroid-Pre-phase) followed by 3 pulses of Dexamethasone (20 mg/day for 4 days each) combined with Vincristine, Daunorubicin, Pegylated Asparaginase, and Methotrexate as phase 1 of induction chemotherapy. It was felt that the glucocorticoid component of this therapeutic regimen will constitute an effective therapy for TIN as well. Serum creatinine levels started to decrease 3 days after initiation of Dexamethasone, decreasing to 80 μmol/L 8 weeks later (Figure 3). Eosinophilia, hematuria, and proteinuria resolved by 2 weeks, 4 weeks, and 6 weeks, respectively, after initiation of steroid therapy. The patient traveled abroad for stem cell transplantation after receiving the second phase of induction therapy.
Discussion
Initial evaluation of this patient was focused on determining the etiology of the visible hematuria and acute kidney injury. Urinary tract infection, coagulopathies, structural lesions of the urinary tract, and nephrolithiasis had already been excluded by initial laboratory tests and radiological imaging of the urinary tract 1 week earlier.
Glomerular diseases such as IgA nephropathy, crescentic glomerulonephritis, and post-streptococcal glomerulonephritis were considered as a cause of macroscopic hematuria and acute kidney injury. Heavy glomerular hematuria in IgA nephropathy can also induce acute kidney injury through oxidative stress and tubulotoxicity. This possibility was also contemplated. On the other hand, absence of edema, normal blood pressure, eosinophilia, pyuria, and low-grade proteinuria after recent intake of antibiotics also raised the suspicion of drug-induced TIN. Red blood cell casts, once considered a hallmark of glomerular pathology, have also been described in acute TIN, caused by red blood cell entry into tubular lumens where they admix with Tamm-Horsfall glycoprotein [4]. Conversely, macroscopic hematuria is uncommon with the use of modern antibiotics (in contrast to Methicillin-induced TIN, which has long been considered prototypical of drug-induced TIN) [3,5,6]. Renal histopathology not only confirmed TIN but also disclosed a remarkable finding of florid interstitial hemorrhages. Interstitial hemorrhage is usually seen in rather different clinical settings, such as renal infarction, infectious nephritides (eg, Herpes simplex virus, Adenovirus, Hantavirus, Rickettsia), ANCA-related vasculitis, trauma, coagulopathies, and in kidney transplant patients, acute cellular/antibody-mediated rejection. It appears that the interstitial inflammation was severe enough to lead to a significant breach in the integrity of the walls of the interstitial blood vessels and tubular basement membranes. This resulted in generous extravasation of red blood cells from the peri-tubular capillaries into the interstitium, causing widespread interstitial hemorrhages. The hemorrhages then extended into the renal tubules, producing visible hematuria and red blood cell casts [7]. Raised interstitial pressure secondary to the inflammatory process may have facilitated the entry of red blood cells into the tubules through disrupted tubular basement membranes. Interestingly, Ferrari et al described a case of acute interstitial nephritis after Amoxicillin, featuring intratubular red blood cells and red blood cell casts on renal biopsy [8]. By contrast, frank interstitial hemorrhages were not seen. Interstitial hemorrhages have been reported in patients with IgA nephropathy who were concurrently taking oral anticoagulants [9,10]. Direct immunofluorescence studies ruled out IgA nephropathy in our patient. Slightly raised anti-streptolysin O antibodies’ titer probably indicated previous streptococcal infection. However, there was no serological or histo-logical evidence of post-infectious glomerulonephritis: serum complement levels were normal, the glomeruli did not show any proliferative/crescentic change, and immunofluorescence studies precluded immune complex mediated glomerulopathies. Ultrastructural examination of glomeruli was desirable to rule out the possibility of co-existing thin basement membrane disease or Alport syndrome, but electron microscopy is not available at our institution. Nevertheless, the presence of many red blood cells in renal tubules, but not in the Bowman’s spaces, was suggestive of a ‘tubular’ rather than ‘glomerular’ origin of hematuria.
An important consideration in our patient, excluded by immunohistochemical workup, was renal interstitial infiltration by leukemic cells. This has been documented in 83% of autopsy cases of acute lymphoblastic leukemia, although interstitial hemorrhages have not been described [11]. Renal interstitial infiltration by leukemic cells can cause acute kidney injury, either by causing vascular stasis without any structural injury to the nephrons, or by inducing TIN [12–15]. However, in both these situations, immunohistochemical staining is positive for leukemic cells. BK virus infection has been reported to induce hemorrhagic tubulointerstitial nephritis in patients with acute leukemia. This possibility was also excluded by negative SV40 staining.
In summary, antecedent exposure to Amoxicillin-Clavulanate, systemic eosinophilia, abundance of eosinophils and plasma cells in the interstitial infiltrate, and exclusion of other renal pathologies in our patient suggested Amoxicillin-Clavulanate-induced hemorrhagic tubulointerstitial nephritis. The hematuria started within 3 weeks of exposure to Amoxicillin-Clavulanate, which is consistent with the time course of drug-induced TIN. An analysis of more than 150 cases of drug-induced TIN by Rosert et al revealed that renal manifestations developed within 3 weeks of starting the inciting drug in about 80% of patients [6]. The etiology is a delayed hypersensitivity immune reaction driven by antigen-reactive T cells; therefore, the reaction is idiosyncratic, not related to the dose of Amoxicillin-Clavulanate or duration of therapy [3,16,17]. We believe that glucocorticoids given during the steroid-pre-phase and as a part of induction therapy promoted the resolution of interstitial inflammation and recovery of renal function, thereby avoiding the need for dialysis. Glucocorticoids are often employed to treat severe interstitial nephritis that persists despite discontinuation of the inciting agent.
Conclusions
This patient with T cell acute lymphoblastic leukemia had Amoxicillin-Clavulanate-induced TIN featuring extensive interstitial hemorrhages in conjunction with visible hematuria and red blood cell casts. Antibiotic-related TIN should be considered in patients presenting with these features, simulating glomerulonephritis, vasculitis, or infectious interstitial nephritis. The key is to follow an open-minded diagnostic approach, moving from a broader to a narrower differential diagnosis list.
Figures
References:
1.. Nast CC, Medication-induced interstitial nephritis in the 21st century: Adv Chronic Kidney Dis, 2017; 24(2); 72-79
2.. Michel DM, Kelly CJ, Acute interstitial nephritis: J Am Soc Nephrol, 1998; 9(3); 506-15
3.. Praga M, González E, Acute interstitial nephritis: Kidney Int, 2010; 77(11); 956-61
4.. Fogazzi GB, Ferrari B, Garigali G, Urinary sediment findings in acute interstitial nephritis: Am J Kidney Dis, 2012; 60(2); 330-32
5.. González E, Gutiérrez E, Galeano C, Early steroid treatment improves the recovery of renal function in patients with drug-induced acute inter-stitial nephritis: Kidney Int, 2008; 73(8); 940-46
6.. Rossert J, Drug-induced acute interstitial nephritis: Kidney Int, 2001; 60(2); 804-17
7.. Sigala JF, Biava CG, Hulter HN, Red blood cell casts in acute interstitial nephritis: Arch Intern Med, 1978; 138(9); 1419-21
8.. Ferrari B, Fogazzi GB, Garigali G, Messa P, Acute interstitial nephritis after amoxycillin with hematuria, red blood cell casts and hematuria-induced acute tubular injury: Clin Nephrol, 2013; 80(2); 156-60
9.. Martín Cleary C, Moreno JA, Fernández B, Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury: Nephrol Dial Transplant, 2010; 25(12); 4103-6
10.. Escoli R, Santos P, Andrade S, Carvalho F, Dabigatran-related nephropathy in a patient with undiagnosed IgA nephropathy: Case Rep Nephrol, 2015; 2015; 298261
11.. Xiao JC, Walz-Mattmüller R, Ruck P, Horny HP, Kaiserling E, Renal involvement in myeloproliferative and lymphoproliferative disorders: A study of autopsy cases. Gen Diagn Pathol, 1997; 142(3–4); 147-53
12.. Dahlbeck SW, Tome M, Kagan AR, Cooper R, The role of radiation therapy in children with acute lymphoblastic leukemia kidney infiltration: Med Pediatr Oncol, 2001; 37(5); 477-478, doi: 10.1002/mpo.1234
13.. Biro E, Szikszay E, Pethő-Orosz P, Bigida L, Balla G, Szabó T, Acute interstitial nephritis in T-cell leukemia in a pediatric patient: Pediatr Int, 2016; 58(9); 940-42
14.. Nakajima T, Tsujimoto I, Ujihira N, Sezaki R, Tubulointerstitial nephritis caused by chronic lymphocytic leukemia: Intern Med, 2015; 54(6); 685-86
15.. Suzuki Y, Katayama K, Ishikawa E, Granulomatous interstitial nephritis due to chronic lymphocytic leukemia: a case report: BMC Nephrol, 2017; 18(1); 348
16.. Raghavan R, Eknoyan G, Acute interstitial nephritis – a reappraisal and update: Clin Nephrol, 2014; 82(3); 149-62
17.. Kodner CM, Kudrimoti A, Diagnosis and management of acute interstitial nephritis: Am Fam Physician, 2003; 67(12); 2527-34
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250