18 April 2022: Articles 
Transient Complete Blindness Due to Metformin-Associated Lactic Acidosis (MALA) Reversed with Hemodialysis
Mistake in diagnosis, Management of emergency care, Rare disease, Adverse events of drug therapy, Clinical situation which can not be reproduced for ethical reasons
Libardo Rueda Prada12ABCDEFG*, Lindsey Knopps3EF, Igor DumicDOI: 10.12659/AJCR.935730
Am J Case Rep 2022; 23:e935730
Abstract
BACKGROUND: Metformin-associated lactic acidosis (MALA) is a relatively rare adverse effect of metformin therapy. It usually occurs in patients with metformin overdose or in those with underlying acute and/or chronic conditions resulting in impaired lactate metabolism. Among these, patients with acute kidney injury, heart failure, sepsis, and cirrhosis are the most vulnerable to MALA, even in the setting of appropriate therapy. The most common symptoms of MALA include nausea, vomiting, diarrhea, encephalopathy, hypothermia, respiratory failure, and hypotension. Blindness is a dramatic symptom that has been rarely reported with MALA.
CASE REPORT: We report a case of 78-year-old woman with history of type 2 diabetes mellitus with nephropathy for which she was treated with metformin and insulin. She developed nausea, non-bloody emesis, and watery diarrhea, which led to dehydration, anion gap metabolic acidosis due to hyperlactatemia, and acute kidney injury (AKI). She was hospitalized for i.v. hydration and further management when she suddenly developed blindness. The diagnostic work-up ruled out central causes and her symptoms resolved briefly after continuous renal replacement therapy (CRRT) was initiated, confirming the diagnosis of MALA.
CONCLUSIONS: By reporting this case, we wish to increase awareness about MALA symptoms, its diagnosis, and the importance of early recognition and initiation of treatment among clinicians involved in the care of patients with chronic kidney disease (CKD) who take metformin for diabetes mellitus. Although rare, this metformin adverse effect can present dramatically and can be distressing for both patient and treating team.
Keywords: Acidosis, Lactic, Blindness, Dialysis, Metformin, Acute Kidney Injury, Diabetes Mellitus, Type 2, diarrhea, Female, Humans, Hypoglycemic Agents, Male, Nausea, Renal Dialysis
Background
Metformin is a biguanide used as a first-line medication to treat type 2 diabetes mellitus. It has multiple advantages, including low cost, weight neutrality, low risk for hypoglycemia, and anti-inflammatory and cardioprotective effects. It is a well-tolerated medication with a good safety profile [1]. Historically, metformin was not recommended for use in patients with an eGFR <60 mL/min/1.73 m2. In 2016, the US Food and Drug Administration (FDA) recommended the use of metformin even in patients with eGFR of 30 to 60 mL/min/1.73 m2 [2]. This resulted in the addition of more than one million patients with CKD who are now on therapy with metformin. This change has likely contributed to an increase in cases of metformin-associated adverse effects [3]. The most common adverse effects from metformin are gastrointestinal, including nausea, diarrhea, and flatulence. Other reported adverse effects are cyanocobalamin deficiency, asthenia, headache, and hepatitis [4]. MALA occurs mostly in patients with underlying co-morbidities resulting in impaired metformin and lactic acid metabolism. The incidence of MALA is estimated to be 1–10 per 100 000 [5,6], and is usually reported in patients with AKI who have impaired metformin clearance [6]. MALA can be life threatening. Its mortality historically has been reported to be as high as 50% [7]. However, newer reports suggest a much lower, but still unacceptably high, mortality rate of 17.2% [6]. Transient blindness has been rarely reported as a symptom of MALA [8–12].
Case Report
A 78-year-old woman presented to the emergency room complaining of 2 weeks of progressively worsening non-bloody diarrhea with approximately 5 bowel movements per day. Three days prior to presentation, she developed left lower-quadrant dull abdominal pain accompanied by nausea and non-bloody vomiting. She did not have fever, headache, cough, shortness of breath, jaw claudication, joint pain, or any visual disturbances. She lived in Wisconsin, had not travelled outside of the state recently, and reported no sick contacts. She was immunized against COVID-19 with the Moderna mRNA vaccine 4 months before presentation. Her past medical history was significant for poorly controlled type 2 diabetes mellitus (A1C 8.4%), hypertension, CKD 3b A2, depression, hypertriglyceridemia, and anemia with CKD. Her surgical history included bilateral cataract surgery 10 years prior. She was compliant with her medications up until the day prior to admission, when she could not take them due to nausea and vomiting. These included: aspirin 81 mg daily, atorvastatin 40 mg daily, famotidine 20 mg twice daily as needed for heartburn, glargine insulin 18 units subcutaneous at bedtime, aspart insulin 20 units subcutaneous 3 times daily before meals, metformin 1000 mg twice daily, metoprolol tartrate 25 mg twice daily, losartan 100 mg daily, torsemide 20 mg daily, and sertraline 150 mg daily. She denied recent medication changes and she had been taking metformin since 2005. She was a former smoker and denied a history of alcohol or illicit drug use. The family history was negative for cerebrovascular disease or vasculitides.
Initial vital signs showed temperature of 36.3°C, blood pressure 165/67 mmHg, heart rate 104 beats per minute, and respiratory rate 16 breaths per minute. Oxygen saturation was normal on ambient air. A physical exam showed an alert, pale woman in no distress. Her pupils were round, equal 2–3 mm, and reactive to light and accommodation. Mucous membranes were dry. Heart sounds were regular with tachycardia, and no murmurs or rubs. Lungs were clear to auscultation. An abdominal exam was significant for mild tenderness to palpation of the left lower quadrant, with no guarding or rebound tenderness. Her neurological exam was normal.
The initial laboratory work-up showed leukocytosis 10.2×109/L with neutrophilia and lymphopenia, hemoglobin 10.6 g/dL, mild thrombocytosis 438×109/L, lactate 6.4 mmol/L, sodium 135 mmol/L, hypochloremia 86 mmol/L, hyperkalemia 6.2 mmol/L, anion gap elevated metabolic acidosis with bicarbonate 6 mmol/L, anion gap 43, no osmolar gap, glucose 269 mg/dL, blood urea nitrogen 83 mg/dL, creatinine 8.47 mg/dL (baseline creatinine 1.2–1.4 mg/dL), normal liver transaminases and total bilirubin, creatine kinase 110 U/L, venous blood gas pH <6.80, pCO2 17 mmHg, and beta-hydroxybutyrate 1.8 mmol/L (ref range 0.0–0.5 mmol/L), and the SARS CoV-2 polymerase chain reaction (PCR) test negative. Urinalysis showed no signs of infection, but was positive for glucose 500 mg/dL and ketones 80 mg/dL. A computed tomography (CT) scan of the abdomen and pelvis without intravenous (i.v.) contrast showed extensive colonic diverticula with some inflammatory change in the fat around the descending colon, which could represent mild diverticulitis. There was no free air or abscess collection.
We administered i.v. ceftriaxone and Flagyl, 2 L NaCl 0.9% fluid bolus, 300 mEq bicarbonate bolus, and bicarbonate drip (150 mEq of bicarbonate in 1 L free water at 250 cc/h). A repeat lactate showed an increase to 9.6 mmol/L. New imaging of the abdomen and pelvis with i.v. contrast ruled out the possibility of acute mesenteric ischemia. The patient was admitted to the Intensive Care Unit and an insulin drip was initiated with addition of a 2-L NaCl 0.9% fluid bolus. Three hours after the initial presentation, the patient developed confusion, lethargy, hypothermia (32.2°C), and sudden painless bilateral vision loss described as “floating blue clouds.” Her blood pressure and heart rate remained stable. A repeat detailed physical exam showed pupils were equal at 3–4 mm with no light pupillary reaction, and absent blink to threat. The remaining neurological exam results were normal. A repeat abdominal exam showed no change.
A CT head was negative for acute abnormalities. Emergent ophthalmology evaluation revealed preserved extraocular motility, no evidence of retinopathy, macular edema, acute glaucoma, or embolic phenomena, and confirmed no pupillary reaction to light and absent blink to threat. Given her risk factors for cerebrovascular disease, cortical blindness was considered. Magnetic resonance imaging (MRI) of the brain revealed no acute abnormalities, which ruled out acute stroke. Repeated blood work 7 h after initial presentation showed worsening lactic acid of 14.2 mmol/L, sodium 138 mmol/L, hypochloremia 92 mmol/L, hyperkalemia 6.0, bicarbonate <5, anion gap unable to calculate, and glucose 264. A new venous blood gas analysis 9 h after presentation showed pH 6.87 and pCO2 29 mmHg, along with worsening lactic acid of 16.1 (Figure 1). Due to persistent metabolic disturbances and development of new neurologic deficit, MALA with vision loss was suspected. The patient was initiated on emergent CRRT. She developed hypo-tension at that point and norepinephrine infusion was initiated. Transthoracic echocardiogram (TTE) revealed preserved left ventricular ejection fraction of 64% without segmental wall motion abnormalities, and no evident intracardiac mass or thrombus. This actively ruled out a cardiogenic etiology of shock. Volatile serum screen revealed high acetone at 18 mg/dL and was negative for methanol, ethanol, and isopropanol.
Two hours after CRRT initiation, metabolic acidosis and vision started to improve, and hours later her vision and mental status returned to her baseline. Hypothermia gradually resolved. Bicarbonate infusion was discontinued. Lactic acid normalized.
Insulin drip was transitioned to subcutaneous insulin. She was weaned off norepinephrine within 7 h. An infectious diseases work-up (blood and urine cultures) remained negative. Abdominal exam remained benign, and no recurrence of diarrhea was noted. Forty-eight hours after initial presentation, empiric antibiotics were discontinued as sepsis was deemed unlikely. Dialysis was discontinued after 18 h. Creatinine improved to 2.93 mg/dL and the hyperkalemia resolved. She was discharged home in stable condition with an adjusted insulin regimen and indefinite discontinuation of metformin.
Discussion
Pyruvate is produced from glucose during glycolysis, and subsequently enters the Krebs cycle if oxygen is available to the cells. Under anaerobic conditions, pyruvate is unable to enter mitochondria and is reduced to lactate by lactate dehydrogenase [13]. Lactate is metabolized by the liver (around 70%), while 30% is excreted by the kidneys. Hence, diseases that affect the liver and kidneys, mainly cirrhosis and acute renal failure, will lead to lactate accumulation and hyperlactatemia. There are 2 distinct types of hyperlactatemia: type A and type B. Type A occurs in the setting of tissue hypoperfusion such as severe sepsis, trauma, cardiogenic shock, and/or seizure. Type B hyperlactatemia is independent of tissue oxygenation and most commonly is caused by medications that interfere with lactate metabolism [13]. While both types can be associated with hemodynamic instability and hypotension, it is important to note that hypotension is the cause of type A hyperlactatemia, while it is a consequence of type B [13].
Type B hyperlactatemia can be caused by many medications. For example, use of beta 2 agonist can increase lactic acid in some patients due to increased pyruvate production [13]. Propylene glycol is metabolized to lactate, and it was described as a cause of lactic acidosis in patients who were treated with lorazepam infusion (for alcohol withdrawal), since 80% of volume in lorazepam infusion is propylene glycol [13]. Furthermore, linezolid can cause hyperlactatemia B due to inhibition of mitochondrial protein synthesis, which is similar to nucleoside reverse transcriptase inhibitors used for treatment of patients infected with human immunodeficiency virus. Propofol can cause hyperlactatemia as part of propofol-related infusion syndrome (constellation of hyperlactatemia, rhabdomyolysis, renal failure, hepatomegaly, and hyperlipidemia) due to inhibition of the electron transport chain [13].
There are several theories to explain development of MALA. Glucose conversion to lactate in the splanchnic bed of the small intestine can be increased by metformin [14]. The MALA mechanism also involves metformin’s mechanism of action, which is inhibition of gluconeogenesis via inhibition of mitochondrial respiratory chain complex 1. During gluconeogenesis, lactate is transformed into glucose. When this is inhibited, lactate accumulates, leading to hyperlactatemia [15,16]. However, it is rare to have significant hyperlactatemia with a therapeutic metformin concentration and in patients with normal liver function. Usually, another factor needs to be present for significant hyperlactatemia to develop, such as renal failure (impaired metformin excretion) or liver failure (impaired metformin metabolism) or significant tissue hypoperfusion (severe sepsis, cardiogenic shock), which lead to increased lactate production as a result of anaerobic metabolism [5,13,17,18].
Careful review of medications that our patient was taking did not reveal another potential culprit or significant interaction that could have led to an increased metformin concentration. We hypothesize that her initial diarrhea was an adverse effect of metformin or it was a self-limiting viral gastrointestinal infection. Regardless of the etiology of the diarrhea, it led to dehydration. This, coupled with her taking losartan and torsemide, led to AKI, which led to metformin accumulation and development of MALA. Symptoms that developed later, just before admission and shortly afterwards (nausea, vomiting, encephalopathy, hypotension, and blindness), were attributed to MALA. We ruled out a stroke with a negative CT and MRI brain imaging, cardiogenic shock was ruled out by normal TTE findings, and infection was ruled out by negative imaging and cultures. Monocular blindness, unlike bilateral blindness, as in our case, is the most common presentation of transient ischemic attack associated with transient visual symptoms [19].
Blindness in our patient was reversed by dialysis, which supported the diagnosis of MALA. MALA mimicking sepsis is an important differential diagnosis to exclude, as treatment for these 2 conditions is significantly different.
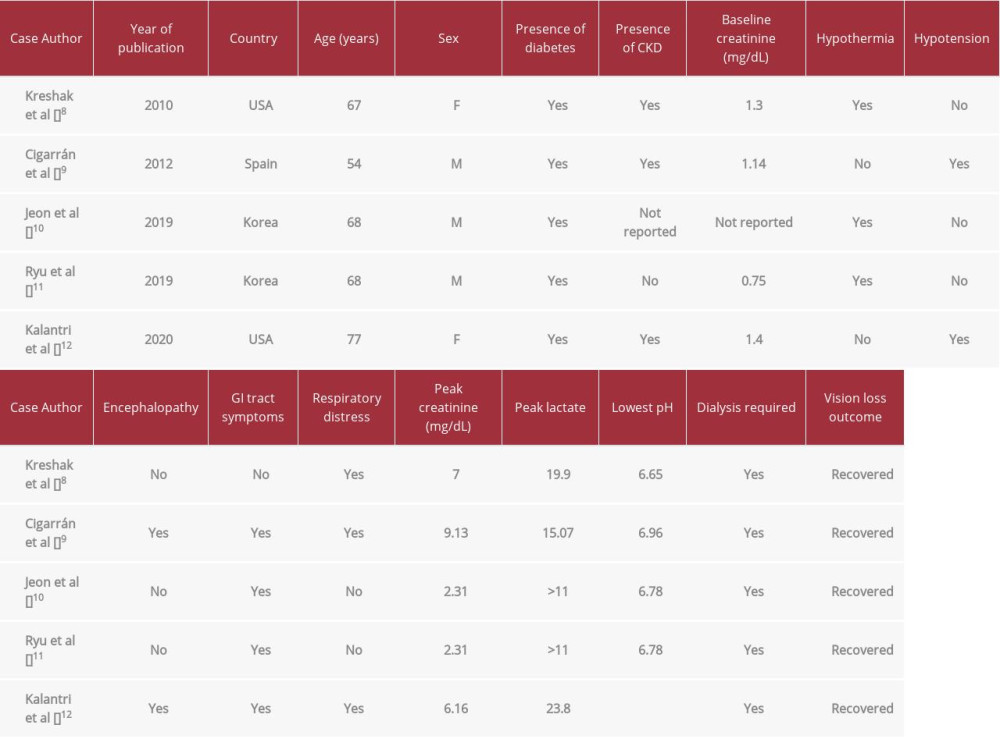
Blindness in patients who developed MALA has rarely been reported. A search of the PubMed database using the key words “transient vision loss” and “acidosis” and “metformin-associated lactic acidosis” yielded only 5 cases [8–12]. The clinical features of these patients are presented in Table 1. Two of these case reports may have been of the same patient based on information obtained from case report (see highlighted rows in Table 1). In all of these cases, patients were treated with dialysis, and vision loss was transient and reversible following correction of acidosis. However, the patient in the case report described by Jeon et al recovered vision even before hemodialysis was initiated, after he was started on bicarbonate infusion.
Due to the rarity of this manifestation of MALA, human studies are impossible to conduct to better understand its patho-physiology. However, some animal models suggest that retinal horizontal cells are particularly sensitive to low pH. During the state of acidemia, these cells might interrupt transmission of signals to the visual neurons, with consequent blindness [20,21].
Episodes of blindness have been reported in other types of acidosis, such as ketoacidosis or alcohol-related acidosis [22,23]. Interestingly, it has not been described in patients with lactic acidosis from severe sepsis, so another factor (eg, metformin metabolites or ketones), in addition to low pH, may contribute to impaired function of retinal cells.
Timely dialysis seems to be very effective in the treatment of these patients. A recent report by Kinoshita et al [24] showed good outcome even in a patient who had multiple risk factors for poor outcome and presented with very low pH (6.6), high lactate (above 18 mmol/L), and extremely elevated metformin concentration (77.5 mg/L, normal value below 2 mg/L).
Treatment of MALA mainly consists of supportive measures such as bicarbonate infusion and i.v. fluids to correct metabolic acidosis and to normalize lactic acid and pH levels. As MALA is frequently mistaken for lactic acidosis due to severe sepsis, these patients frequently receive inappropriate antibiotics [6]. Some patients with MALA, especially those with complications such as blindness, may need renal replacement therapy (RRT). The pre-dialysis level of serum lactate is a marker of mortality in MALA and was significantly higher in non-survivors (median 22.5 mmol/L) than in survivors (median 17 mmol/L, p-value <0.01) [6]. Modalities of RRT used to treat patients with MALA include peritoneal dialysis, intermittent RRT, prolonged intermittent RRT, and continuous RRT [6]. A patient with MALA should undergo dialysis without interruption until their lactate level is less than 3 mmol/L [6]. Vision loss seems to improve as soon as metabolic acidosis is corrected [25].
Conclusions
Although rare, MALA is a serious and dramatic adverse effect of metformin, even at therapeutic doses. Therefore, it should be suspected and considered in any patient on metformin therapy who presents with lactic acidosis and blindness. Its incidence is predicted to rise over the next several years given the recent relaxation in eGFR restrictions for metformin, and an increase in the prevalence of CKD. Prompt RRT has been demonstrated to be pivotal for recovery and significantly lowers the morbidity and mortality associated with this condition. RRT in this scenario reverses blindness, metabolic de-arrangements, and hemodynamic compromise rapidly, in just a few hours. Such a response effectively rules out the most important differential diagnosis in this group of patients with cortical blindness.
References:
1.. Kansagara D, Qaseem A, Wilt TJ, Guidelines on glycemic targets for persons with type 2 diabetes: JAMA, 2018; 320(18); 1937
2.. , FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function, 2016 Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf
3.. Crowley MJ, Diamantidis CJ, McDuffie JR, Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: A systematic review: Ann Intern Med, 2017; 166(3); 191-200
4.. , Metformin Hydrochloride, drug information Available at: https://www.micromedexsolutions.com
5.. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE, Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: Cochrane Database Syst Rev, 2010; 2010(4); CD002967
6.. Yeh HC, Ting IW, Tsai CW, Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy: A systematic review of case reports and case series: BMC Nephrol, 2017; 18(1); 229
7.. Seidowsky A, Nseir S, Houdret N, Fourrier F, Metformin-associated lactic acidosis: A prognostic and therapeutic study: Crit Care Med, 2009; 37(7); 2191-96
8.. Kreshak AA, Clark RF, Transient vision loss in a patient with metformin-associated lactic acidosis: Am J Emerg Med, 2010; 28(9); 1059.e5-7
9.. Cigarrán S, Rodriguez ML, Pousa M, Transient vision loss in a patient with severe metformin-associated lactic acidosis: QJM, 2012; 105(8); 781-83
10.. Jeon JW, Choi W, Kim HR, Transient blindness in a patient with severe metformin-associated lactic acidosis (MALA): Electrolyte Blood Press, 2019; 17(1); 16-20
11.. Ryu S, Oh SK, Son SH, Reversible acute blindness in suspected metformin-associated lactic acidosis: J Emerg Med, 2019; 57(5); e153-56
12.. Kalantri P, Sahu A, Kalantri A, A case report on metformin-associated lactic acidosis and transient blindness: Cureus, 2020; 12(7); e9325
13.. Blohm E, Lai J, Neavyn M, Drug-induced hyperlactatemia: Clin Toxicol (Phila), 2017; 55(8); 869-78
14.. Bailey CJ, Wilcock C, Day C, Effect of metformin on glucose metabolism in the splanchnic bed: Br J Pharmacol, 1992; 105(4); 1009-13
15.. , Global, regional, and national comparative risk assessment of 84 behavioral, environmental, and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017: Lancet, 2018; 392(10159); 1923-94
16.. Renda F, Mura P, Finco G, Metformin-associated lactic acidosis requiring hospitalization. A national 10-year survey and a systematic literature review: Eur Rev Med Pharmacol Sci, 2013; 17; 45-49
17.. Vecchio S, Giampreti A, Petrolini VM, Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy: Clin Toxicol (Phila), 2014; 52(2); 129-35
18.. Spiller HA, Sawyer TS, Toxicology of oral antidiabetic medications: Am J Health Syst Pharm, 2006; 63(10); 929-38
19.. Lavallée PC, Cabrejo L, Labreuche J, Spectrum of transient visual symptoms in a transient ischemic attack cohort: Stroke, 2013; 44(12); 3312-17
20.. Osborne NN, Casson Rj, Wood JP, Retinal ischemia: Mechanisms of damage and potential therapeutic strategies: Prog Retin Eye Res, 2004; 23(1); 91-147
21.. Barnes S, Merchant V, Mahmud F, Modulation of transmission gain by protons at the photoreceptor output synapse: Proc Natl Acad Sci USA, 1993; 90; 10081-85
22.. Bockus LB, Asad ZUA, Chaudhary AMD, Awab A, Reversible blindness as presenting manifestation of severe diabetic ketoacidosis: Am J Med Sci, 2019; 357(2); 164-67
23.. Yanagawa Y, Kiyozumi T, Hatanaka K, Reversible blindness associated with alcoholic ketoacidosis: Am J Ophthalmol, 2004; 137(4); 775-77
24.. Kinoshita H, Yanai M, Ariyoshi K, A patient with metformin-associated lactic acidosis successfully treated with continuous renal replacement therapy: A case report: J Med Case Rep, 2019; 13(1); 371
25.. Sirtori CR, Pasik C, Re-evaluation of a biguanide, met-formin: Mechanism of action and tolerability: Pharmacol Res, 1994; 30(3); 187
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250