17 April 2022: Articles 
Complete Plaque Regression in Patients with Peyronie’s Disease After Multimodal Treatment with Antioxidants: A Report of 2 Cases
Unknown etiology, Unusual clinical course, Mistake in diagnosis, Unusual or unexpected effect of treatment, Congenital defects / diseases, Educational Purpose (only if useful for a systematic review or synthesis)
Gianni Paulis1ABEF*, Giovanni De Giorgio2EFDOI: 10.12659/AJCR.936146
Am J Case Rep 2022; 23:e936146
Abstract
BACKGROUND: Peyronie’s disease is a chronic inflammatory disease involving the tunica albuginea of the penile corpora cavernosa. Conservative medical treatment includes oral therapy, intralesional injections, and physical treatment. No cases of Peyronie’s disease with complete plaque regression after conservative medical treatment are described in the literature.
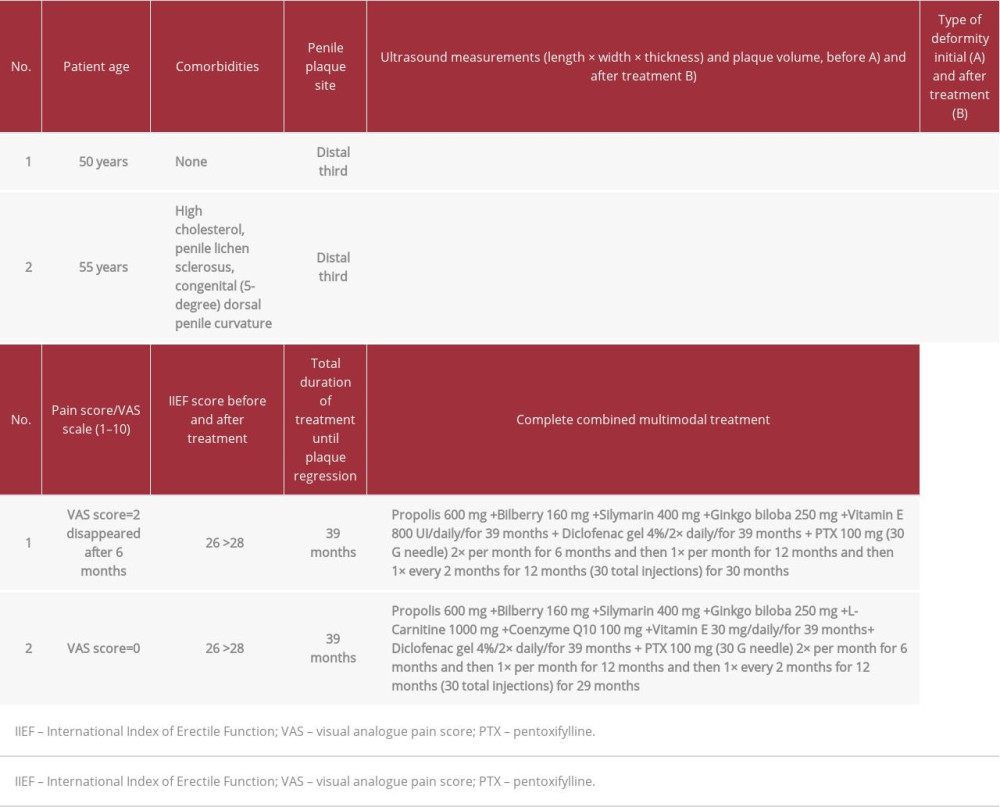
CASE REPORT: Case 1: A 50-year-old man presented with penile pain, combined penile curvature (left dorso-lateral), a palpable nodule, and normal penile rigidity. The patient underwent multimodal therapy with oral antioxidants, topical diclofenac gel, and periodic perilesional penile injections with pentoxifylline. At follow-up after over 3 years of multimodal treatment, the patient no longer had penile deformity or pain. Ultrasound imaging did not show any area affected by disease and plaque could no longer be observed. Case 2: A 55-year-old man presented with penile lichen sclerosus, dorsal penile curvature with onset about 6 months before, penile plaque, no penile pain, and normal penile rigidity. He underwent multimodal therapy with oral antioxidants, topical diclofenac gel, and periodic perilesional penile injections with pentoxifylline. At follow-up after over 3 years of multimodal treatment, he presented a dorsal curvature of the penis of about 5°, similar to his condition prior to the onset of congenital penile curvature. Ultrasound no longer showed plaque.
CONCLUSIONS: This study showed that multimodal combined antioxidant treatment had satisfactory results. However, to accomplish this, we deem it essential to have a correct ultrasound assessment that is performed with a sufficiently advanced machine by an operator with extensive experience in the field.
Keywords: Antioxidants, Oxidative Stress, Penile Induration, pentoxifylline, Combined Modality Therapy, Diclofenac, Humans, Male, Pain
Background
Peyronie’s disease (PD) is a chronic inflammatory disease involving the tunica albuginea of the corpora cavernosa and leads to the formation of fibrous or calcified plaque, which causes penile deformity (eg, curvature, hourglass deformity). Symptoms of PD are penile deformity (94%), penile pain (20–70%), erectile dysfunction (over 30%) [1,2], and depression (48%) [3]. The etiology of the disease has not been clarified, but the most credited theory is that it is caused by trauma [4]. The consequent accumulation of fibrin in the site of the trauma is thought to cause an inflammatory process, resulting in an overproduction of fibrogenic cytokines and free radicals [5–8]. Conservative medical treatment includes oral therapy, intralesional injections, and physical treatment: colchicine, vitamin E, potassium para-aminobenzoate, tamoxifen, antioxidants, phosphodiesterase 5 inhibitors, injections with verapamil, corticosteroids, clostridium histolyticum collagenase (CHC/Xiaflex-Xiapex), interferon-α2b, hyaluronic acid, pentoxifylline, iontophoresis, extracorporeal shock wave therapy, vacuum devices, and penile traction devices [9,10]. Surgical treatment is indicated when the disease has been stable for at least 6 to 12 months, or if the patient can no longer have complete sexual intercourse due to severe curvature or severe erectile dysfunction [11–13]. Because of our experience in multimodal treatment of PD using antioxidants [14], we present 2 cases of patients with PD who achieved complete regression of disease and penile plaque with this treatment. Combined or multimodal treatment also implies the association of therapeutic agents administered in various ways, such as orally and by injection, without excluding the association of physical therapy with the aid of devices, such as iontophoresis, extenders, vacuum devices, and extracorporeal shock wave therapy. Multimodal treatment aims to achieve better results than those obtained with a single substance or therapy.
The aim of this report is to present to urologists and andrologists 2 patients with PD who achieved the regression of plaque after receiving treatment with multimodal therapy. To the best of our knowlegde, this is the first article to describe complete plaque regression achieved in patients with PD. Compared to previous conservative treatments described in the literature, our multimodal treatment included several active substances that can stop the inflammatory process by countering the oxidative stress that plays an essential role in the pathogenesis of PD.
Case Reports
CASE 1:
A 50-year-old man presented to our clinic in November 2013 with concerns of penile pain during erection and penile curvature with onset about 9 months before. He had no associated medical conditions and was a nonsmoker. The visual analog pain scale score was 2 (score from 0 [low] to 10 [high]). A subjective assessment of erection, evaluated using the International Index of Erectile Function (IIEF) questionnaire, yielded a score of 26 (normal score range is from 26 to 30). The penile deformity consisted of a dorsal curvature, with a 15° angle, and a lateral left curvature of 30°. A physical examination on penile palpation revealed a nodule measuring about 15 mm in length. The patient underwent a physical examination and penile Doppler ultrasound with alprostadil 10 mcg to induce an erection. The volume of the penile plaque was measured in 3 dimensions using the ellipsoid formula: vo lume=0.524×length×width×thickness [15,16].
Cavernous artery flow and end-diastolic velocity were normal: peak systolic velocity was 65 cm/s (bilaterally) and end-diastolic velocity was 0 cm/s (bilaterally). The penile plaque was located dorsally and at the distal third of the penis, its ultrasound aspect was heterogeneous, and it measured 13.4×9.78×2.94 mm (volume=202 mm3); within the plaque there was a calcification measuring 3.42×3.43×1.50 mm (Figure 1).
In order to obtain the patient’s informed consent, he was informed of the required length of treatment owing to the presence of a chronic (and not acute) inflammatory disease. The patient did not consent to the publication of his penis images, even if anonymous. Beginning in January 2014, after giving the informed consent to publish his clinical data, the patient underwent the following treatment: combined therapy with antioxidants, including oral propolis 600 mg, bilberry 160 mg, silymarin 400 mg, ginkgo biloba 250 mg, and vitamin E 800 UI daily; topical diclofenac gel 4% twice daily; and perilesional penile injection pentoxifylline 100 mg (1 ampule) with a 30 G needle every 2 weeks for 6 months. At follow-up at the end of the first treatment cycle, the patient had an increase in the IIEF-questionnaire score from 26 to 28. A disappearance of the dorsal curve was then observed, as well as the persistence of the lateral left curvature, with an angle reduced to 15°. Penile pain was no longer present. On palpation, a smaller nodule was detected.
The penile ultrasound showed nodule dimensions of 8.17×7.70×3.19 mm (volume=105 mm3), and within the nodule a calcification measured 2.83×2.44×1.5 mm (Figure 2). The plaque had therefore decreased in size by 48.0% compared with its initial volume. Furthermore, the calcification within the plaque had diminished in size.
Because of the treatment result, together with the patient, we decided to continue the same oral treatment for the next 12 months, but with penile peri-plaque injections of pentoxifyl-line 100 mg only once a month. At follow-up at the end of the second treatment cycle after about 18 months of therapy, the IIEF score was 28, and the left curvature was observed to have disappeared. Penile palpation detected the nodule was further reduced in size. An ultrasound showed nodule dimensions of 5.04×5.18×2.00 mm (volume=27 mm3), and within the nodule the calcification measured 1.88×1.25×1.22 mm (Figure 3). The plaque was therefore 86.6% smaller than its initial volume, and even the calcification was further reduced in size.
Because of the further excellent response to treatment, we decided to continue the same oral treatment for the next 12 months, but with penile peri-plaque injections once every 2 months.
At the end of the third cycle of treatment, after about 30 months of therapy, the patient underwent a similar follow-up. The IIEF score was unchanged at 28. Follow-up revealed the absence of penile curvature. Palpation no longer detected the presence of the nodule. Ultrasound showed plaque dimensions of 4.05×4.69×1.87 mm (volume=19 mm3), with the disappearance of the internal calcification (Figure 4). The plaque had therefore shrunk by 90.6%, compared with its initial volume.
Because of the significant response to treatment, we decided to continue the same therapy for just 6 more months and permanently suspend the peri-plaque pentoxifylline injections. At follow-up after 6 months of exclusive oral and topical diclofenac treatment, and after a little over 3 years of multimodal treatment with antioxidants, the patient no longer reported any penile deformity or pain. The IIEF score was still 28. Palpation did not reveal the presence of any nodule, ultrasound did not show any area affected by disease, and plaque could no longer be observed (Figure 5, Table 1). We therefore decided to suspend the treatment. The patient did not report any adverse effects after the treatment. The same ultrasound machine was used at every examination (Philips HD 15) and the examination was performed by the same operator every time.
CASE 2:
A nonsmoking 55-year-old man with penile lichen sclerosus presented to our clinic in March 2018. He did not report any penile pain, but had penile curvature, with onset about 6 months before. The patient presented a dorsal curvature of the penis. Penile color Doppler ultrasound, with injection of alprostadil 10 mcg to induce erection, revealed a curvature by goniometric measurement of about 32°. The IIEF score was 26 (normal range: 26–30). On physical examination, we detected by palpation a nodule measuring 12 to 15 mm in length at the distal third of the penis. Cavernous artery flow and end-diastolic velocity were normal: peak systolic velocity was 78 cm/s on the right and 75 cm/s on the left and end-diastolic velocity was 0 cm/s (bilaterally). The penile plaque, located at the distal third of the penis, had a heterogeneous (iso-hyperechoic) appearance and dimensions of 14.3×8.49×2.57 mm (length×width×thickness); its volume was 163 mm3 (Figure 6, Table 1) [15,16].
In order to obtain the patient’s informed consent, he was informed of the necessary length of treatment owing to the presence of a chronic (and not acute) inflammatory disease. The patient did not consent to the publication of his penis images, even if anonymous. Therefore, after receiving the patient’s informed consent to publish his clinical data, we began the following treatment in April 2018: combined therapy with the antioxidants oral propolis 600 mg, bilberry 160 mg, silymarin 400 mg, ginkgo biloba 250 mg, L-carnitine 1000 mg, coenzyme Q10 100 mg, and vitamin E 30 mg daily; topical diclofenac gel 4% twice daily; and peri-plaque penile injection of pentoxifylline 100 mg with a 30 G needle every 2 weeks for 6 months.
After completing the first cycle of treatment, the patient underwent follow-up. The IIEF score was unchanged at 26. On palpation, we observed the nodule was slightly reduced in size. We then observed the angle of the dorsal curve was reduced to 25°. Penile ultrasound showed plaque dimensions of 11.9×7.39×2.35 mm (volume=108 mm3) (Figure 7). The volume of the plaque was 33.7% smaller than its initial volume.
Because of the good response to the first multimodal treatment, we decided to schedule a second cycle of oral and topical treatment for 12 months, using the same agents and doses, but reducing the frequency of peri-plaque injections to once a month. At follow-up at the end of the second cycle of treatment the IIEF score was still 26. We then observed a further reduction in the angle of the dorsal curve to 20°. On palpation, the nodule was scarcely observable. Penile ultrasound detected plaque dimensions of 7.08×5.32×2.4 mm (volume=47 mm3) (Figure 8), which was 71.1% smaller than its initial volume.
Considering the good response to the second cycle of combined treatment, we agreed with the patient to do a third cycle of treatment for another 12 months, using the same substances and the same doses and further reducing the frequency of peri-plaque penile injections to 1 penile injection every 2 months.
The patient underwent follow-up opon completion of the third treatment cycle. We no longer detected a penile nodule during penile palpation. We then observed a further reduction in the angle of the dorsal curve to 15°. Ultrasound showed plaque dimensions of 4.80×3.65×2.38 mm (volume=22 mm3) (Figure 9), which was a reduction of 86.6%.
In view of the improvement in the patient’s clinical situation, we decided to suspend the peri-plaque injections and to continue the oral therapy and home topical therapy with the same substances and the same daily doses for another 6 months (fourth cycle of treatment). At follow-up after 6 months of oral and topical (diclofenac) antioxidant treatment, and after approximately 3 years and 3 months of multimodal treatment, the IIEF score was 28 (normal range: 26–30), penile palpation did not detect any nodules, and ultrasound examination no longer showed any plaque (Figure 10A, 10B).
The angulation of the dorsal curve had greatly improved compared to the previous follow-up, with an angle of 5 degrees, quite similar to the patient’s condition prior to disease (congenital dorsal penile curvature) (see Table 1).
Our multimodal treatment with antioxidants was therefore suspended. The patient did not report adverse effects from the treatment. The same ultrasound machine was used at initial presentation and in the follow-up examinations (Philips Affinity 70 G), and the ultrasound examination was always performed by the same doctor.
Discussion
The possibility that PD may improve after multimodal treatment with antioxidants has already been confirmed in the literature, both in terms of a reduction in the affected area (plaque) and improvement in the corresponding deformity [14,17]. To the best of our knowledge, this is the first report in which complete plaque regression was achieved in patients with PD. In the literature, there is only 1 experimental study on rats in which complete plaque regression was achieved [18]. There is only 1 published study with a long period of multimodal therapy, but the duration of treatment did not exceed 18 months [19]. Considering the long period of time (over 3 years) required for disease regression in our patients, we must keep in mind that chronic inflammatory diseases are inherently very long lasting, and therefore an adequate period of treatment is needed to stop progression of the disease and gradually have a reabsorption of plaque.
We are treating many patients with PD in our practice with a similar multimodal therapy, and some of these patients, although they dropped out of treatment because in their opinion it was too long, showed a reduction in plaque and penile curve. Other patients are treated with the same treatment, and we expect they will end the therapy with excellent results. We believe this is possible because of the properties of the antioxidants combined in the multimodal treatment, which we know are able to stop the inflammatory process by countering the oxidative stress that plays an essential role in the pathogenesis of PD. All the substances we used in the combined therapy (propolis, bilberry, coenzyme Q10, silymarin, ginkgo biloba, vitamin E, L-carnitine, and diclofenac) inhibit activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and block the production of pro-inflammatory cytokines [8]. Regarding the use of diclofenac in multimodal treatment, we considered from the start that topical treatment with this substance would be useful in treating PD in patients, not only due to its well-established painkilling and anti-inflammatory properties, but also for its free-radical scavenging activity and its action against the pro-inflammatory cytokine cascade, including production of factor NF-κB. Another reason we use diclofenac is its proven capacity of being absorbed topically, not only in subcutaneous but even in subfascial tissues. A study by Radermacher et al (1991) showed that diclofenac in gel form, if administered topically, can penetrate into the articular capsule of the knee, which we know is much thicker than the tunica albuginea of the corpora cavernosa of the penis [20].
Although we kept the entire oral therapy and topical treatment with gel unchanged for the entire treatment period, at each subsequent treatment cycle, we reduced the frequency of peri-plaque injections with pentoxifylline 100 mg. We made this decision because the disease had an interrupted progression already after the first cycle of treatment and even presented signs of partial regression on physical examination and diagnostic imaging. Considering, moreover, that PD has a traumatic genesis and even microtrauma can have serious consequences in patients genetically predisposed to the disease, after every cycle of treatment we reduced the number of penile injections because, objectively, injections also constitute a microtrauma for the tissues, although very limited (we used a very thin needle and performed peri-plaque injections).
Many researchers view ultrasound imaging of PD as incapable of providing accurate measurement of plaque [21,22]. However, we believe that if the ultrasound examination is carried out using a cutting-edge ultrasound machine and the procedure is performed by an operator who is highly experienced in this type of disease, the diagnostic information that can be obtained (measurement of the plaque) is very helpful in the assessment of patients and in subsequent follow-ups to correctly evaluate treatment outcome [23].
Conclusions
Although this study was conducted on a limited number of patients, it showed that our multimodal combined antioxidant treatment made it possible to achieve very satisfactory results. However, to accomplish this, we believe it is essential to have a proper ultrasound assessment, which needs to be performed with a sufficiently advanced machine by an operator with extensive experience in the field. We believe the goal achieved in these patients with PD is useful for urology and andrology clinical practice.
Therefore, despite the limited number of patients, we decided to publish this experience and hope that new randomized studies will be conducted on this topic using control groups and a larger number of patients.
Figures
References:
1.. Pryor JP, Ralph DJ, Clinical presentations of Peyronie’s disease: Int J Impot Res, 2002; 14(5); 414-17
2.. Weidner W, Schroeder-Printzen I, Weiske WH, Sexual dysfunction in Peyronie’s disease: An analysis of 222 patients without previous local plaque therapy: J Urol, 1997; 157(1); 325-28
3.. Nelson CJ, Diblasio C, Kendirci M, The chronology of depression and distress in men with Peyronie’s disease: J Sex Med, 2008; 5(8); 1985-90
4.. Devine CJJ, Somers KD, Jordan GH, Proposal: trauma as a cause of Peyronie’s lesion: J Urol, 1997; 157; 285-90
5.. Somers KD, Dawson DM, Fibrin deposition in Peyronie’s disease plaque: J Urol, 1997; 157(1); 311-15
6.. Sikka SC, Hellstrom WJG, Role of oxidative stress and antioxidants in Peyronie’s disease: Int J Impot Res, 2002; 14(5); 353-60
7.. El-Sakka AI, Salabas E, Dinçer M, The pathophysiology of Peyronie’s disease: Arab J Urol, 2013; 11(3); 272-77
8.. Paulis G, Romano G, Paulis L, Recent pathophysiological aspects of Peyronie’s disease: Role of free radicals, rationale, and therapeutic implications for antioxidant treatment – literature review: Adv Urol, 2017; 2017; 4653512
9.. Natale C, McLellan DM, Yousif A, Review of intralesional collagenase clostridium histolyticum injection therapy and related combination therapies in the treatment of Peyronie’s disease (an update): Sex Med Rev, 2021; 9(2); 340-49
10.. Yousif A, Natale C, Hellstrom WJG, Conservative therapy for Peyronie’s disease: A contemporary review of the literature: Curr Urol Rep, 2021; 22(2); 6
11.. Chung E, Ralph D, Kagioglu A, Evidence-based management guidelines on Peyronie’s disease: J Sex Med, 2016; 13(6); 905-23
12.. Hatzichristodoulou G, Osmonov D, Kübler H, Contemporary review of grafting techniques for the surgical treatment of Peyronie’s disease: Sex Med Rev, 2017; 5(4); 544-52
13.. Osmonov D, Ragheb A, Ward S, ESSM position statement on surgical treatment of Peyronie’s disease: Sex Med, 2021; 10(1); 100459
14.. Paulis G, Combination therapy (in the treatment of Peyronie’s disease): Peyronie’s disease A comprehensive guide, 2015; 97-105, Switzerland, Springer International Publishing
15.. Lee JS, Chung BH, Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical pros-tatectomy specimens: Urol Int, 2007; 78(4); 323-27
16.. Eri LM, Thomassen H, Brennhovd B, Accuracy and repeatability of prostate volume measurements by transrectal ultrasound: Prostate Cancer Prostatic Dis, 2002; 5(4); 273-78
17.. Paulis G, Barletta D, Turchi P, Efficacy and safety evaluation of pentoxifylline associated with other antioxidants in medical treatment of Peyronie’s disease: A case-control study: Res Rep Urol, 2016; 8; 1-10
18.. Kwon KD, Choi MJ, Park JM, Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie’s disease: BJU Int, 2014; 114(6); 926-36
19.. Paulis G, Cavallini G, Giorgio GD, Long-term multimodal therapy (vera-pamil associated with propolis, blueberry, vitamin E and local diclofenac) on patients with Peyronie’s disease (chronic inflammation of the tunica albuginea). Results of a controlled study: Inflamm Allergy Drug Targets, 2013; 12(6); 403-9
20.. Radermacher J, Jentsch D, Scholl MA, Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease: Br J Clin Pharmacol, 1991; 31(5); 537-41
21.. Hatzimouratidis K, Eardley I, Giuliano F, EAU guidelines on penile curvature: Eur Urol, 2012; 62(3); 543-52
22.. McCauley JF, Dean RC, Diagnostic utility of penile ultrasound in Peyronie’s disease: World J Urol, 2020; 38(2); 263-68
23.. Parmar M, Masterson JM, Masterson TA, The role of imaging in the diagnosis and management of Peyronie’s disease: Curr Opin Urol, 2020; 30(3); 283-89
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250