22 November 2022: Articles 
Lisinopril-Induced Small Bowel Angioedema: An Unusual Cause of Severe Abdominal Pain
Challenging differential diagnosis, Unexpected drug reaction, Rare disease
Brooks W. JohnsonDOI: 10.12659/AJCR.937895
Am J Case Rep 2022; 23:e937895
Abstract
BACKGROUND: Angiotensin-converting enzyme inhibitors (ACE-I) are one of the most frequently prescribed classes of medications with the rare adverse effect of angioedema, and isolated small bowel angioedema is a small subset of these cases. Isolated angioedema of the small bowel is a rare adverse effect of this commonly prescribed medication, and it mimics, symptomatically and radiographically, several other illnesses and is often misdiagnosed. While ACE-I are thought to be safe, the risk of angioedema is approximately 0.7%. Isolated small bowel angioedema is often not diagnosed in a timely manner, and misdiagnosis leads to significant morbidity in afflicted patients.
CASE REPORT: We present the case of a 63-year-old woman with angioedema of the small bowel causing abdominal pain, nausea, vomiting, and diarrhea. Computed tomography demonstrated small bowel edema and ascites. The patient had been taking lisinopril for 7 years prior to presentation and had previously been seen by multiple physicians for abdominal pain. A diagnosis of ACE-I-induced small bowel angioedema was made and lisinopril therapy was immediately stopped. Her symptoms improved with cessation of lisinopril, and follow-up imaging showed resolution of the angioedema 3 months later.
CONCLUSIONS: The course of ACE-I-induced angioedema is unpredictable and often overlooked as a cause of abdominal pain. It commonly presents soon after starting ACE-I therapy, but can present years after therapy initiation, as in this case. Significant morbidity, including unnecessary exploratory laparotomy, is associated with misdiagnosis of ACE-I-induced angioedema of the small bowel. Prompt recognition and cessation of the offending drug is crucial but often delayed.
Keywords: Allergy and Immunology, angioedema, Gastroenterology, Internal Medicine, Lisinopril, Female, Humans, Middle Aged, Abdominal Pain, Angiotensin-Converting Enzyme Inhibitors, Abdomen
Background
Angiotensin-converting enzyme inhibitors (ACE-I) are one of the most frequently prescribed classes of medications, currently used by approximately 40 million people globally [1]. ACE-I are first-line antihypertensives in patients with cardiovascular disease and diabetes mellitus, and are the most prescribed class of antihypertensives overall [2–4]. While ACE-I are thought to be safe medications, they carry the risk of angioedema in approximately 0.7% of users [5]. Angioedema is a serious condition that can prove fatal and therefore requires rapid recognition and treatment, despite its relative rarity [4]. ACE-I-induced angioedema occurs when excess bradykinin builds up due to decreased breakdown by ACE, leading to increased vascular permeability and swelling [3]. Most patients who develop ACE-I-induced angioedema present with facial and oropharyngeal swelling [5]. However, there have been several previous case reports identifying angioedema of the small bowel either in isolation or in conjunction with facial and oropharyngeal edema [1,5–19]. We present an additional case of ACE-I-induced angioedema with characteristic radiologic findings of angioedema of the small bowel, in the absence of facial and oropharyngeal involvement. Of note, our patient had been taking lisinopril for 7 years prior to the onset of symptoms. It is known that angioedema can develop at any time following ACE-I therapy induction; however, it is generally expected to develop during the first 5 years [20,21].
Case Report
Our patient was a 63-year-old White woman with a past medical history of hyperlipidemia, hypothyroidism, diverticulitis, and essential hypertension, which was treated for 7 years with lisinopril. Her current dose of lisinopril was 30 mg, with no recent changes. She reported no history of alcohol, tobacco, or drug use. She presented to the Emergency Department (ED) following a 3-day history of severe abdominal pain. She described her pain as a 9–10/10 in intensity and sharp in character. The pain had steadily increased in severity over the previous 3 days and was nearly unbearable on presentation. With the onset of this pain, she began to have high-volume light-green watery diarrhea. She was experiencing significant nausea, anorexia, chills, sweating, and flushing. Importantly, she denied any symptoms of difficulty breathing or swelling of her face, lips, or tongue.
She described having similar pain episodes over the past few months but denied any prior diarrhea with these previous episodes. Previous computed tomography (CT) imaging 1 month prior was negative for radiographic evidence of pathology.
Her vital signs on arrival to the ED were a heart rate of 78 beats per min, blood pressure of 138/78 mmHg, temperature of 36°C, respiratory rate of 17 breaths per min, and an O2 saturation of 97% on room air. Physical examination showed an uncomfortable appearing woman with a normal facial and oropharyngeal examination, a diffusely swollen and exquisitely tender abdomen with normoactive bowel sounds, and a normal skin examination. Her laboratory studies were remarkable for a C-reactive protein level of 59.5 mg/dL and a hemoglobin level of 16.1 g/dL. She had a C1 esterase-inhibitor level of 81 mg/dL, C4 level of 33 mg/dL, and C1 esterase-inhibitor antigen level of 22 mg/dL, which were all within the reference range. All other laboratory tests, including white blood cells, lactate, and electrolytes, were normal. CT imaging demonstrated multiple distal small bowel loops with edematous bowel wall thickening, which was suggestive of medication-induced angioedema (Figures 1, 2).
A stool pathogen panel was taken and returned negative; therefore, a presumed diagnosis of ACE-I-induced angioedema was made. Her lisinopril was immediately stopped, and therapy was begun with diphenhydramine, famotidine, and methylprednisolone. The next morning, she was feeling significantly better and was able to be discharged with outpatient follow-up with her primary care physician and the Allergy/ Immunology Department.
Of note, 3 days after discharge, the patient presented to the ED with a chief concern of abdominal pain, but the nausea, vomiting, and diarrhea had resolved. She was treated symptomatically and discharged home. Since that time, there were no reported episodes of abdominal pain. Approximately 3 months following her initial presentation, she underwent follow-up CT imaging, which revealed no small or large bowel wall thickening or obstruction, with complete resolution of the angioedema of the small bowel (Figure 3).
Discussion
Angioedema is an uncommon but well-described adverse effect of ACE-I and most commonly involves the oropharynx and periorbital region [22]. There are limited case reports of isolated ACE-I-induced angioedema of the bowel in the literature, and the disease is underdiagnosed and underreported [5]. Previous literature review of patients with ACE-I angioedema has demonstrated that the duration of ACE-I treatment at the onset of angioedema ranged from 1 day to 8 years, with a median of 6 months [23].
Angiotensin-converting enzyme (ACE), the target of ACE-I medications, is identical to the kininase II enzyme, which is responsible for the metabolism and inactivation of bradykinin [24]. Therefore, pharmacological inhibition of ACE leads to increased levels of bradykinin. Previous research has shown increased levels of bradykinin in patients with hereditary angioedema as well as acquired angioedema, with significantly elevated levels of bradykinin during acute episodes. We sought to differentiate our patient’s diagnosis of angioedema as ACE-I-induced rather than hereditary. Hereditary angioedema occurs in the vast majority of cases due to a deficiency or mutation in the C1 esterase inhibitor [25,26]. Hereditary angioedema therefore generally manifests with low C1 esterase inhibitor and C4 levels, particularly during flares [27].
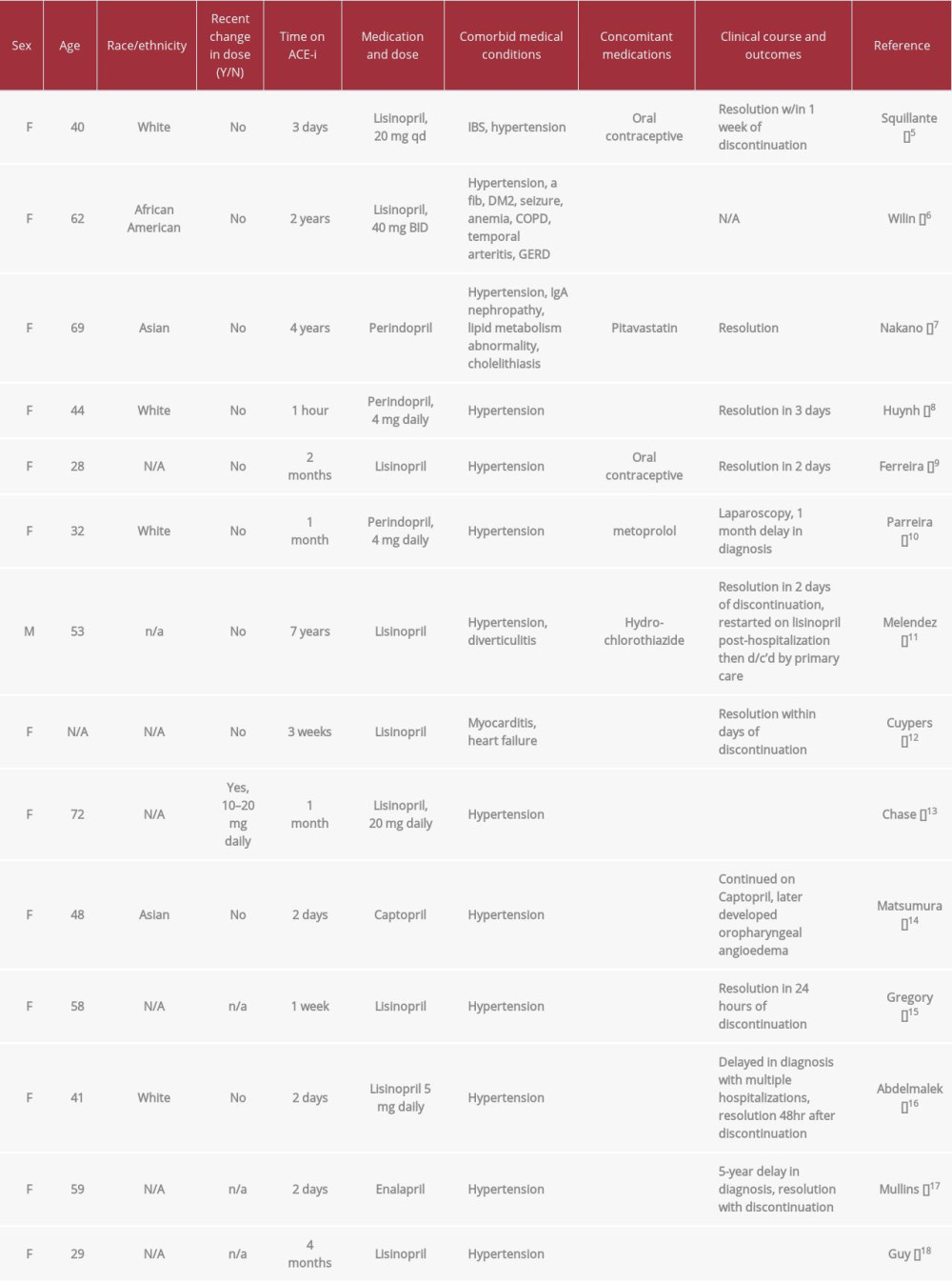
Angioedema is a self-limiting condition characterized by increased capillary permeability with subsequent extravasation of fluid causing tissue edema of the effected organ. A review of reported cases of angioedema induced by ACE-I from 1966 to 1997 showed an incidence of 0.1% to 0.5% in patients taking an ACE-I, and only a fraction of those cases were angioedema of the bowel [28]. Unlike other drug-induced reactions causing angioedema, the incidence of angioedema in patients taking ACE-I is usually inconsistent and not temporally associated with the timing of ACE-I ingestion. As such, ACE-I is frequently missed as a cause of angioedema, and patients may continue to take ACE-I for management of their hypertension after one or multiple bouts of angioedema. One retrospective study even reported that physicians often attributed angioedema to several causes not related to ACE-I use, even after multiple recurrences. Continuing ACE-I after the first occur-rence of angioedema is associated with an increased rate of serious morbidity [29]. A review of published cases of isolated small bowel angioedema demonstrates that it disproportionately affects woman, there is a wide range of time from initiating ACE-I therapy to first symptoms, and the disease does not seem to discriminate based on age (Table 1).
Patients with angioedema of the small bowel present with abdominal pain, nausea, and vomiting. Less commonly, patients present with diarrhea and/or ascites. Patients are overwhelmingly female and usually between the ages of 40 to 70 years. Previous case reviews report an increased incidence in African American women. Diagnosis is made by imaging, often CT, which shows edema of the stomach, duodenum, jejunum and/or ileum with accompanying ascites [5,30]. The presence of ascites is strongly associated with angioedema of the bowel and decreases the probability of infectious etiologies [30].
The differential diagnosis for a patient with undifferentiated, acute, diffuse abdominal pain is wide. The most common etiologies to consider include infectious, ischemic, inflammatory, functional, and traumatic. If paracentesis is performed, it shows nonspecific inflammatory findings [31]. In up to 15% of cases, findings are diagnosed as ischemic bowel, and patients are taken to the operating room for unnecessary laparotomy or laparoscopy [28].
Furthermore, although it is uncommonly reported in the literature, even after patients have stopped lisinopril, they can experience recurrent episodes of abdominal pain in the immediate period after discontinuation [31].
The patient presented in this report had been taking lisinopril for approximately 7 years prior to diagnosis. She had no previous episodes of oropharyngeal or periorbital swelling but did describe previous episodes of abdominal pain for which she visited the ED. During her previous workup, there had been no indication of bowel edema, ascites, or other objective signs of angioedema of the bowel, although her symptoms were identical to those when the diagnosis of ACE-I-induced angioedema was made. Following diagnosis and discontinuation of ACE-I, she once again experienced abdominal pain shortly after discharge and was treated symptomatically.
Conclusions
Lisinopril, and other ACE-I, are likely an underdiagnosed cause of abdominal pain, as the symptoms are nonspecific. Furthermore, previous reports of ACE-I-induced bowel angioedema have been reported in patients with limited use of ACE-I (less than 1 year in most cases), which may lead a clinician to believe that ACE-I-induced angioedema of the bowel is less likely a culprit in patients with long-time use of ACE-I. In turn, patients may undergo invasive testing and continue taking ACE-I with significant associated morbidity.
This case report demonstrates 3 uncommon features that are important to recognize in a patient with ACE-I-induced angioedema of the bowel. First, the patient had been taking lisinopril for 7 years prior to diagnosis and discontinuation of the offending medication. Second, the patient had multiple episodes of severe abdominal pain, without radiographic evidence of angioedema prior to diagnosis. Lastly, the patient had an episode of severe, self-resolving abdominal pain following discontinuation of the ACE-I.
Figures
References:
1.. Kostis WJ, Shetty M, Chowdhury YS, Kostis JB, ACE Inhibitor-induced angioedema: A review: Curr Hypertens Rep, 2018; 20(7); 55
2.. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF, Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use?: J Am Coll Cardiol, 2018; 71(13); 1474-82
3.. Wilkerson RG, Winters ME, Angiotensin-converting enzyme inhibitor-induced angioedema: Emerg Med Clin North Am, 2022; 40(1); 79-98
4.. Vasekar M, Craig TJ, ACE inhibitor-induced angioedema: Curr Allergy Asthma Rep, 2012; 12(1); 72-78
5.. Wilin KL, Czupryn MJ, Mui R, ACE inhibitor-induced angioedema of the small bowel: A case report and review of the literature: J Pharm Pract, 2018; 31(1); 99-103
6.. Squillante MD, Trujillo A, Norton J, ACE inhibitor induced isolated angioedema of the small bowel: A rare complication of a common medication: Case Rep Emerg Med, 2021; 2021; 8853755
7.. Nakano Y, Kuwahara R, Miyamoto S, Spontaneous rapid improvement of small intestinal edema: Gastroenterology, 2022; 162(4); e12-13
8.. Huynh TNA, Hua L, Smalberger JA, James J, ACE inhibitor induced intestinal angioedema: ANZ J Surg, 2022 [Online ahead of print]
9.. Ferreira TA, Alves MR, Oliveira AMP, A rare cause of abdominal pain: Intestinal angioedema: J Med Cases, 2021; 12(4); 138-40
10.. Parreira R, Amaral R, Amaral L, ACE inhibitor-induced small bowel angioedema, mimicking an acute abdomen: J Surg Case Rep, 2020; 2020(10); rjaa348
11.. Melendez M, Grosel JM, ACE inhibitor-induced angioedema causing small bowel obstruction: JAAPA, 2020; 33(8); 28-31
12.. Cuypers S, Van Meerbeeck S, De Pauw M, ACE inhibitor-induced angioedema of the small intestine: A case report: Acta Cardiol, 2011; 66(5); 645-48
13.. Chase MP, Fiarman GS, Scholz FJ, MacDermott RP, Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor: J Clin Gastroenterol, 2000; 31(3); 254-57
14.. Matsumura M, Haruki K, Kajinami K, Takada T, Angioedema likely related to angiotensin converting enzyme inhibitors: Intern Med, 1993; 32(5); 424-26
15.. Gregory KW, Davis RC, Images in clinical medicine. Angioedema of the intestine: N Engl J Med, 1996; 334(25); 1641
16.. Abdelmalek MF, Douglas DD, Lisinopril-induced isolated visceral angioedema: Review of ACE-inhibitor-induced small bowel angioedema: Dig Dis Sci, 1997; 42(4); 847-50
17.. Mullins RJ, Shanahan TM, Dobson RT, Visceral angioedema related to treatment with an ACE inhibitor: Med J Aust, 1996; 165(6); 319-21
18.. Guy C, Cathébras P, Rousset H, Suspected angioedema of abdominal viscera: Ann Intern Med, 1994; 121(11); 900
19.. Matsumura M, Haruki K, Kajinami K, Takada T, Angioedema likely related to angiotensin converting enzyme inhibitors: Intern Med, 1993; 32(5); 424-26
20.. Banerji A, Blumenthal KG, Lai KH, Zhou L, Epidemiology of ACE inhibitor angioedema utilizing a large electronic health record: J Allergy Clin Immunol Pract, 2017; 5(3); 744-49
21.. Montinaro V, Cicardi M, ACE inhibitor-mediated angioedema: Int Immunopharmacol, 2020; 78; 106081
22.. Israili ZH, Hall WD, Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology: Ann Intern Med, 1992; 117(3); 234-42
23.. Agostoni A, Cicardi M, Cugno M, Angioedema due to angiotensin-converting enzyme inhibitors: Immunopharmacology, 1999; 44(1–2); 21-25
24.. Nussberger J, Cugno M, Amstutz C, Plasma bradykinin in angio-oedema: Lancet, 1998; 351(9117); 1693-97
25.. Billebeau A, Fain O, Launay D, Hereditary angioedema with and without C1-inhibitor deficiency in postmenopausal women: J Clin Immunol, 2021; 41(1); 163-70
26.. Wilkerson RG, Moellman JJ, Hereditary angioedema: Emerg Med Clin North Am, 2022; 40(1); 99-118
27.. Stepaniuk P, Bosonea AM, Pourshahnazari P, The role of C1 inhibitor and complement as acute phase reactants: Are we missing the diagnosis of hereditary angioedema?: Allergy Asthma Clin Immunol, 2021; 17(1); 103
28.. Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ, ACE inhibitor-induced angioedema. Incidence, prevention and management: Drug Saf, 1998; 18(3); 171-88
29.. Brown NJ, Snowden M, Griffin MR, Recurrent angiotensin-converting enzyme inhibitor – associated angioedema: JAMA, 1997; 278(3); 232-33
30.. Benson BC, Smith C, Laczek JT, Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: A case series and literature review: J Clin Gastroenterol, 2013; 47(10); 844-49
31.. Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS, Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: Clinical and imaging findings in 20 patients: Am J Roentgenol, 2011; 197(2); 393-98
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250