18 March 2023: Articles 
Thrombosis with Thrombocytopenia Syndrome (TTS) After ChAdOx1 nCoV-19 Immunization: An Investigative Case Report
Unusual clinical course
Nityanand Jain12ACDEFG*, Piyush Chaudhary3BDE, Amit Shrivastava3BDE, Taranvir Kaur3BDE, Shabjot Kaur3BDE, Harmandeep Singh Brar3BCE, Ravul Jindal3ABCDEFGDOI: 10.12659/AJCR.938878
Am J Case Rep 2023; 24:e938878
Abstract
BACKGROUND: Thrombosis with thrombocytopenia syndrome (TTS), including vaccine-induced immune thrombotic thrombocytopenia (VITT), is an extremely rare adverse effect, mostly seen after initial vaccination with the viral vector-based AstraZeneca-Oxford COVID-19 vaccine. It is characterized by mild to severe thrombocytopenia and venous or arterial thrombosis.
CASE REPORT: Herein, we present a case of an 18-year-old male patient who developed Level 1 TTS (probable VITT) eight days after immunization with the ChADOx1 nCOV-19 vaccine (Covishield; AZ-Oxford). Initial investigations revealed severe thrombocytopenia, hemiparesis, and intracranial hemorrhage, after which the patient was treated conservatively. However, a decompressive craniotomy was performed later due to patient deterioration. One week after surgery, the patient developed bilious vomiting, lower-gastrointestinal bleeding, and abdominal distension. An abdominal CT scan was performed that showed thrombosis of the portal vein with occlusion of the left iliac vein. The patient underwent an exploratory laparotomy followed by resection and anastomosis of the small bowel due to massive gut gangrene. Due to persistent thrombocytopenia after surgery, intravenous immune globulin (IVIG) was administered. The platelet count increased thereafter, and the patient stabilized. He was discharged on the 33rd day after admission and was followed up for a year. No post-hospitalization complications were observed in the follow-up period.
CONCLUSIONS: Although vaccines have been proven to be highly safe and effective to end the Coronavirus Disease 2019 (COVID-19) caused pandemic, there is still a small risk of developing rare complications, including TTS and VITT. Early diagnosis and prompt intervention are key for patient management.
Keywords: ChAdOx1 nCoV-19, COVID-19 Vaccines, Headache, Immunoglobulins, Thrombocytopenia, Venous Thrombosis, Adolescent, Humans, Male, COVID-19, Immunization, Purpura, Thrombocytopenic, Idiopathic, Vaccination
Background
COVID-19 is a SARS-COV-2 (severe acute respiratory syndrome coronavirus-2)-mediated viral infection presenting with varying degrees of clinical severity and symptomology [1]. Since the first documented case of COVID-19 in China in 2019, more than 84 million cases and 1.8 million deaths have been reported globally (as of 1st November 2022). Given its high transmission rate, voluntary mass vaccination plays a crucial role in stemming the transmission of the virus. Additionally, vaccination spikes the immune system, thereby reducing the severity of the disease in infected individuals.
Among the various available COVID-19 vaccines, the recombinant chimpanzee Ad25 vector-based AstraZeneca-Oxford (AZ-Oxford) vaccine is one of the most used vaccines globally, available for use in 182 countries [2]. The AZ-Oxford vaccine manufactured in India is branded as Covishield and was found to be identical in composition and immunogenicity to the parent vaccine [3]. Such vector-based vaccines can induce CD4+ T cell-mediated Th1 immune responses, thereby conferring the recipient stronger immune protection [4]. Malaise, fatigue, headache, myalgia, injection site pain/tenderness are the most reported adverse events following immunization with the AZ-Oxford vaccine [5], most of which can be resolved by taking over-the-counter pain medications like paracetamol and NSAIDs (eg, ibuprofen).
However, in some patients, rather rare but life-threatening adverse effects presenting as thrombocytopenia and thrombosis at unusual localizations have been reported. The condition has been termed vaccine-induced immune thrombotic thrombocytopenia (VITT). Although the exact pathogenetic mechanism and the incidence of the condition are unknown, VITT has been estimated to have a case fatality rate of 23–40% [6]. Since the risk of VITT development following COVID-19 vacci-nation is relatively small (1 case per 26 500 to 127 300 population), the FDA (Food and Drug Administration, USA) has recommended that vector-based vaccines be given only to individuals older than 18 years of age who are otherwise ineligible to receive any other vaccines [6,7].
Clinically, the American Society of Hematology defines VITT as a clinical syndrome presenting 4–42 days after immunization with vector-based vaccines and characterized by the presence of all of the following changes in laboratory and radiological examinations: a) thrombosis at uncommon localizations including cerebral venous sinus thrombosis/splanchnic venous thrombosis; b) mild to severe thrombocytopenia; c) positive antibodies against platelet factor-4 (PF-4) identified by quantitative ELISA (enzyme-linked immunosorbent assay) and d) significantly elevated D-dimer (>4 times upper normal limits) [7]. Thrombosis with thrombocytopenia syndrome (TTS), on the other hand, is a broader category of conditions that can occur due to any cause following COVID-19 vaccination and differs from VITT in that there is no causation by and relationship to anti-PF-4 antibodies [8].
Herein, we present a case of Level 1 TTS (probable VITT) in a male adolescent patient who presented with a rather atypical clinical sequala after immunization with the Covishield vaccine, including presence of intracranial hemorrhage. Given the limited experience (in dealing with VITT) and resource availability in our center, the patient was quickly started on IVIG (intravenous immunoglobulin) to prevent further disease progression. Since the patient received treatment based on the institutional and national guidelines and no new experimental procedures/therapies were investigated, ethics approval was waived by the institutional ethics review committee. Written informed consent to publish the study was provided by the patient.
Case Report
An 18-year-old male patient presented to the emergency department of our hospital (Fortis Hospital Mohali, Punjab, India) in September 2021 with progressively worsening episodes of headaches and vomiting along with left hemiparesis. Eight days before presentation at our hospital, he was actively immunized with his first dose of ChAdOx1 nCOV-19 vaccine (Covishield-Serum Institute of India; AstraZeneca-Oxford) and a day later developed fever. The fever subsided with the use of paracetamol. On the third day after immunization, he was admitted to a local hospital, where blood tests revealed mild thrombocytopenia and he was treated symptomatically. However, he continued to have worsening headaches and hemiparesis, following which he was referred to our department.
On admission, laboratory analysis revealed severe thrombocytopenia with a platelet count of 22 000 cells/µl and D-dimer level of 12.43 µg/mL (normal range <0.46 µg/mL). The patient had no pre-existing conditions, allergies, and did not use any medications. Common causes of thrombocytopenia, including infections (HIV, Hepatitis B and C, Helicobacter Pylori), spontaneous hemarthrosis, bleeding, drug and alcohol use, oncology, exposure to toxic chemicals, past medical history (surgeries and blood transfusions), and family history, were not significant and hence, excluded from the differential diagnosis. The patient was found to be negative for COVID-19 infection, as confirmed by nSARS-COV-2 reverse transcription polymerase chain reaction (RT-PCR) performed on a nasopharyngeal swab sample in our department.
A non-contrast computed tomography (NCCT) of the head revealed a right-sided frontoparietal intracranial hemorrhage (Figure 1). CT cerebral angiogram further revealed the presence of bilateral subarachnoid hemorrhage (SAH) in the high parietal regions. MRI venography of the head was found to be non-significant. By the evening, however, his condition deteriorated, and the patient developed focal seizure, for which antiepileptics were administered. He was intubated and another urgent head CT scan was performed. A massive intracranial hemorrhage with significant midline shift was found. Decompressive craniotomy was performed, and the patient received multiple platelet transfusions. Based on his history and laboratory values, TTS was suspected (probable VITT).
On the 3rd day after admission, prednisolone 60 mg (1 mg/kg/day) was started to treat the thrombocytopenia. Two days later, an increase in the platelet count (72 000 cells/µl) was seen. He was extubated on the 6th day after admission. On the 8th day, he had bloody stools, following which an endos-copy and ultrasound of abdomen was performed. No source of bleeding was identified in the examinations. Laboratory investigations reported leukocytosis (total leucocyte count; TLC 25 680 cells/µl) and platelet count of 150 000 cells/µl (Table 1). The next day, he developed moderate ascites. Antibiotics were administered per standard management protocols.
The patient developed bilious vomiting on the 10th day, following which a Ryle (nasogastric) tube was inserted. The patient did not tolerate feeding through the Ryle tube and developed ileus. After 48 hours, he developed lower gastrointestinal bleeding with blood analysis showing a drop in the platelet count (70 000 cells/µl) and persistent leukocytosis (TLC 21 830 cells/µl). He was started on Nexium (Esomeprazole) infusion. Later in the day, he developed hypotension, tachycardia, and tachypnoea. A slight drop in hemoglobin was noted and he was electively re-intubated and administered positive ino-tropic therapy. An abdominal CT scan showed thrombosis of the portal vein with occlusion of the left iliac vein (Figure 2). Thrombosis in the superior mesenteric vein was also noted.
Consequently, an inferior vena cava (IVC) filter was placed (Figure 3). Anticoagulation therapy could not be initiated due to thrombocytopenia and lower GI tract bleeding. On the 13th day, Fondaparinux 2.5 mg was started once daily due to the high risk of pulmonary embolism. On the next day, the patient developed small-bowel obstruction and underwent an exploratory laparotomy, with a diagnosis of venous gangrene. Resection and anastomosis of the affected small bowel was performed along with a peritoneal lavage for the gangrene (Figure 4). The thrombocytopenia persisted despite administration of multiple platelet transfusions over the following days. In total (including after craniotomy), the patient received 13 SDPs (single-donor platelets) and 1 RDP (random-donor platelets). On the 16th day, after due considerations and consent of the relatives, intravenous immune globulin IVIG (1 g/kg/day) was given. Bleeding stopped after surgery, suggesting that the bleeding was originating from the site of the venous gangrene. In the next 48 hours, a subsequent increase in the platelet count was also noted.
Three days later, tapering of the prednisolone dosage was initiated and Fondaparinux was continued in doses of 2.5 mg twice daily. A Gastrografin (Diatrizoate)-enriched X-ray study of the small bowel revealed no leaks and feeding was re-started with a Ryle tube. A check head CT scan showed an irregular area of cystic encephalomalacia in the right parietal lobe with minimal surrounding residual hemorrhage, which was managed conservatively (Figure 5). The patient slowly improved and was subsequently extubated. He was given continuous feeding via Ryle tube, recommended physiotherapy, continued on Fondaparinux, and was shifted to the ward after 10 days. He was discharged from the hospital on the 33rd day in good health with a prescription for Fondaparinux 2.5 mg twice daily. A brief timeline of events is presented in Figure 6.
Three months later, the IVC filter was removed, and the patient was prescribed total anticoagulation with tablet Eliquis (Apixaban) 5mg twice daily for 6 months. The next 8 months of follow-up were uneventful, and the patient was leading a healthy life, with successful recovery. He was advised against getting vaccinated with a second dose of COVID-19 vaccine.
Discussion
VITT is an extreme immunological reaction that occurs after COVID-19 immunization with viral-vector-based vaccines (Johnson & Johnson/Janssen and AstraZeneca-Oxford) that lead to activation of platelets and the coagulation system, thereby causing venous/arterial thrombosis and/or secondary hemorrhage [9]. Female predominance and presence of thrombi at multiple localizations were initially thought to be associated with VITT [10]. However, the risks for developing VITT also seems to be higher in younger individuals and recipients of a first dose [11]. Persistent and progressively worsening headache along with focal neurological symptoms like visual disturbances have been described as early red flags for suspecting VITT in patients. VITT patients mostly develop thrombosis in the lower extremities and lungs, but unusual sites have also been reported, including splenic, portal, mesenteric, adrenal, cerebral, and ophthalmic veins [12,13]. Laboratory tests suggestive for VITT include platelet count (reduced), D-dimer (elevated), and anti-platelet factor-4 antibodies (elevated).
In our case, a test for PF-4 antibodies could not be performed due to unavailability of the test at our center. The lack of wide public availability of the PF-4 ELISA test has been discussed previously by other authors, and only a few laboratories globally can perform platelet activation assays [14,15]. Additionally, no single ELISA test can accurately detect all cases of VITT, and a second ELISA test has been recommended in case of strong clinical suspicion [9,14]. Reports have highlighted that anti-PF-4 antibodies might be detectable only from 7 to 21 days after vaccination and only in low titers, even in healthy individuals without thrombotic events [16,17]. Furthermore, anti-PF-4 antibodies do not activate platelets in the presence of PF-4 and hence have limited clinical relevance in determining the provocation of clinically evident thrombotic events [16,17]. In fact, it has been shown that there is no correlation between the level of anti-PF-4 antibodies and the level of anti-SARS-CoV-2 IgG, even in patients with confirmed VITT [18]. Based on these arguments, it has been suggested that diagnosis of VITT should be guided by clinical signs and symptoms and not solely depend upon detection of anti-PF-4 antibodies [17].
Nonetheless, for epidemiological purposes, guidelines suggest retrospective detection of anti-PF-4 antibodies using ELISA on stored serum samples [9]. To aid patient classification, multiple interim definitions have been released by health organizations and regulators. Accordingly, based on the Brighton Collaboration’s definition (a non-profit global vaccine safety research network) [19], our patient is categorized as Level 1 TTS and as probable VITT per NICE guidelines [20] due to non-confirmation of anti-PF-4-antibodies.
Non-heparin-based anticoagulation therapy has been recommended as the first-line treatment for VITT in patients with definite or probable diagnosis [9]. In our case, however, anticoagulation therapy was delayed due to active intracranial hemorrhage. The next in-line treatment modality is the use of high-dose IVIG to dampen the immune responses. The delay in starting IVIG in our patient stemmed from financial and logistical issues in securing IVIG. In case of absence of IVIG, steroid treatment has been recommended (our patient was started on prednisolone on day 3 of admission). Regarding platelet transfusions, there is no clear consensus whether they worsen thrombosis in VITT patients or not. Platelet transfusions have been advised for use in patients with life-threatening bleed or those requiring urgent major surgery [9].
Regarding the possibility of the patient undergoing second vaccination against COVID-19, the FDA has suggested that patients with VITT after the first dose of adenoviral vector-vaccine should not be administered a booster dose of adenoviral vector-vaccine [21]. Although a second dose of viral-vector based vaccines has been found to be associated with PF-4-negative VITT, there is no safety information available [9]. Furthermore, it has been demonstrated that VITT antibodies can persist for longer than 3 months in some patients immunized with viral vector-based vaccines (a condition called long VITT) [22]. Similarly, limited data are available on use of inactivated or protein-subunit vaccine boosters. Observational studies have shown that mRNA-based vaccines are well-tolerated in patients with episodes of VITT after primary immunization [23–25]. However, emerging reports have suggested that mRNA-based vaccines may also provoke thrombotic events in patients [26]. In India, since the two most readily available vaccines were Covishield (viral vector) and Covaxin (inactivated), we recommended the patient to avoid getting a second dose, mostly as a precaution.
Timely interventions are essential since patients can deteriorate very quickly. Appropriate public education and medical training for personnel is essential to ensure early detection and management of TTS/VITT cases. Proper surveillance measures are also required to understand the true incidence rate and risk factors for developing TTS/VITT. In India, the National Adverse Events Following Immunization (AEFI) Committee is responsible for compiling such information and reporting it to the government [27]. As of 3rd May 2022, 254 reported cases have received results of the causality assessment (Table 2).
Amongst these cases, two cases were identified as having suffered from TTS – a 21-year-old woman (recovered) and a 22-year-old woman (died) – both vaccinated with Covishield vaccine. Another 18 patients were identified from the database to have had thrombocytopenia, bleeding, thrombosis, or cerebrovascular events. The present case has been reported to the relevant local monitoring authorities per hospital protocols and national guidelines.
Conclusions
The diagnosis of TTS/VITT can be challenging, especially in centers with limited prior experience and resources. Constant patient monitoring and timely symptomatic interventions (both surgical and conservative) are crucial for ensuring patient survivability. IVIG plays a crucial role in stabilizing and improving the condition of patients with TTS/VITT.
Figures
References:
1.. Rana R, Tripathi A, Kumar N, Ganguly NK, A comprehensive overview on COVID-19: Future perspectives: Front Cell Infect Microbiol, 2021; 14(11); 744903
2.. Rashedi R, Samieefar N, Masoumi N, COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts: J Med Virol, 2022; 94(4); 1294-99
3.. , Interim recommendations for use of the ChAdOx1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222, SII Covishield, SK Bioscience), 2021 [cited 2022 November 4]. Available from: https://apps.who.int/iris/rest/bitstreams/1343289/retrieve
4.. Li M, Wang H, Tian L, COVID-19 vaccine development: milestones, lessons and prospects: Signal Transduct Target Ther, 2022; 7(1); 146
5.. Desalegn M, Garoma G, Tamrat H, The prevalence of AstraZeneca COVID-19 vaccine side effects among Nigist Eleni Mohammed memorial comprehensive specialized hospital health workers. Cross sectional survey: PLoS One, 2022; 17(6); e0265140
6.. Leung HHL, Perdomo J, Ahmadi Z, NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia: Nat Commun, 2022; 13(1); 5206
7.. Aleem A, Nadeem AJ, Coronavirus (COVID-19) vaccine-induced immune thrombotic thrombocytopenia (VITT) [Updated 2022 Oct 3]: StatPearls [Internet], 2022, Treasure Island (FL), StatPearls Publishing [cited 2022 November 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570605/
8.. Makris M, Pavord S, Most cases of thrombosis and thrombocytopenia syndrome (TTS) post ChAdOx-1 nCov-19 are vaccine-induced immune thrombotic thrombocytopenia (VITT): Lancet Reg Health Eur, 2021; 12; 100274
9.. Greinacher A, Langer F, Makris M, Vaccine-induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources: J Thromb Haemost, 2022; 20(1); 149-56
10.. Swan D, Enright H, Desmond R, Vaccine-induced thrombosis and thrombocytopenia (VITT) in Ireland: A review of cases and current practices: Thrombosis Update, 2021; 5; 100086
11.. John CV, Kumar R, Sivan AK, Vaccine-induced thrombotic thrombocytopenia (VITT): first report from India: Thromb J, 2022; 20(1); 11
12.. Nazy I, Sachs UJ, Arnold DM, Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology: J Thromb Haemost, 2021; 19(6); 1585-88
13.. Bayas A, Menacher M, Christ M, Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination: Lancet, 2021; 397(10285); e11
14.. Platton S, Bartlett A, MacCallum P, Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination: J Thromb Haemost, 2021; 19(8); 2007-13
15.. Zimmermann S, Federbusch M, Isermann B, Kohli S, Vaccine-induced thrombotic thrombocytopenia: Insights from blood smear: Thromb Haemost, 2021; 121(12); 1696-98
16.. Thiele T, Ulm L, Holtfreter S, Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2: Blood, 2021; 138(4); 299-303
17.. Terpos E, Politou M, Ntanasis-Stathopoulos I, High prevalence of anti-PF4 antibodies following ChAdOx1 nCov-19 (AZD1222) vaccination even in the absence of thrombotic events: Vaccines (Basel), 2021; 9(7); 712
18.. Uzun G, Althaus K, Bakchoul T, No correlation between anti-PF4 and anti-SARS-CoV-2 antibodies after ChAdOx1 nCoV-19 vaccination: N Engl J Med, 2021; 385(14); 1334-36
19.. Chen RT, Monash JB, Updated brighton collaboration case definition for thrombosis with thrombocytopenia syndrome (TTS): Brighton Collaboration, 2021 [cited 2022 November 5]; version 2B. Available from https:////bright-oncollaboration.us/wp-content/uploads/2021/11/TTS-Updated-Brighton-Collaboration-Case-Defintion-Draft-Nov-11-2021.pdf
20.. : COVID-19 rapid guideline: Vaccine-induced immune thrombocytopenia and thrombosis (VITT), 2022, NICE [cited 2022 November 5]; version 5.1. Available fromhttps:////app.magicapp.org/#/guideline/nYP2ZL/section/L4GQAj
21.. , Fact sheet for healthcare providers administering vaccine (vaccination providers), 2022 [cited 2023 January 12]. Available from https://www.fda.gov/media/146304/download?utm_medium=email&utm_source=govdelivery
22.. Gabarin N, Arnold DM, Nazy I, Warkentin TE, Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT): Semin Hematol, 2022; 59(2); 89-96
23.. Schönborn L, Thiele T, Kaderali L, Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient: Blood, 2022; 139(12); 1903-7
24.. Schönborn L, Thiele T, Kaderali L, Greinacher A, Decline in pathogenic antibodies over time in VITT: N Engl J Med, 2021; 385(19); 1815-16
25.. Lacy J, Pavord S, Brown KE, VITT and second doses of COVID-19 vaccine: N Engl J Med, 2022; 386(1); 95
26.. Gurjar H, Dhallu M, Lvovsky D, A rare case of coronavirus disease 2019 vaccine-associated cerebral venous sinus thrombosis treated with mechanical thrombectomy: Am J Case Rep, 2022; 23; e935355
27.. , Causality assessment of 254 reported serious AEFI cases of COVID-19, approved by National AEFI Committee. Government of India, 2022 [cited 2022 November 5]. Available from https://main.mohfw.gov.in/Organisation/Departments-of-Health-and-Family-Welfare/immunization/aefi-reports
Figures
Tables
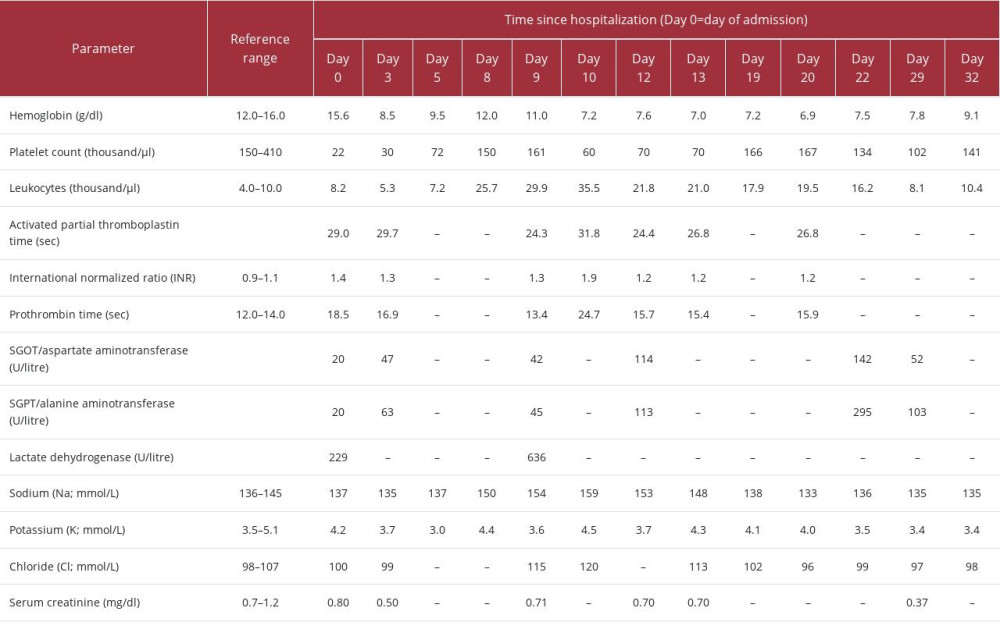 Table 1.. Laboratory analysis for the patient from day of admission to day of discharge.
Table 1.. Laboratory analysis for the patient from day of admission to day of discharge.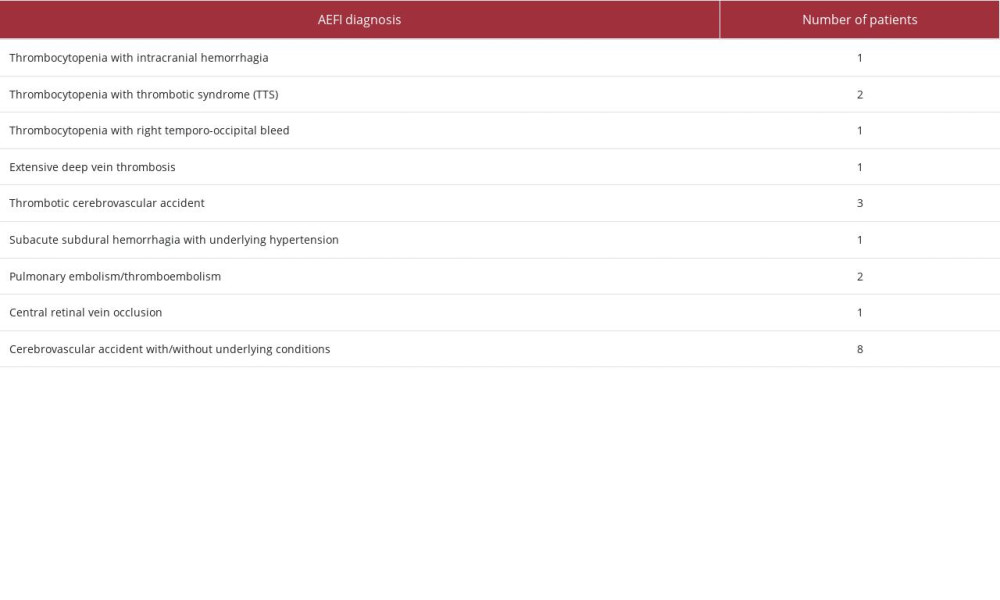 Table 2.. Number of patients reported in AEFI with bleeding, thrombotic, or cerebrovascular events post-COVID-19 immunization.
Table 2.. Number of patients reported in AEFI with bleeding, thrombotic, or cerebrovascular events post-COVID-19 immunization. Table 1.. Laboratory analysis for the patient from day of admission to day of discharge.
Table 1.. Laboratory analysis for the patient from day of admission to day of discharge. Table 2.. Number of patients reported in AEFI with bleeding, thrombotic, or cerebrovascular events post-COVID-19 immunization.
Table 2.. Number of patients reported in AEFI with bleeding, thrombotic, or cerebrovascular events post-COVID-19 immunization. In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








