12 June 2023: Articles 
Surgical Management of Breast Cancer Developing Along the Pathway of a Ventriculoperitoneal Shunt: A Case Report
Unusual or unexpected effect of treatment
Masayuki Saito12ABCDEF, Shinji Kato2AEF, Takashi MaedaDOI: 10.12659/AJCR.939639
Am J Case Rep 2023; 24:e939639
Abstract
BACKGROUND: Ventriculoperitoneal shunts are commonly used in neurosurgery for the treatment of hydrocephalus. This case report details an unusual instance where breast cancer developed along the pathway of an existing ventriculoperitoneal shunt.
CASE REPORT: An 86-year-old woman, who previously underwent ventriculoperitoneal shunt placement for normal-pressure hydrocephalus, visited our hospital upon detecting a mass in her left breast. The physical examination discovered an irregular mass located at the 9 o'clock position of the left breast. Subsequent breast ultrasonography identified a 36 mm mass with indistinct borders, rough margins, and signs of skin infiltration. Invasive ductal carcinoma of a triple-negative subtype was diagnosed through a core-needle biopsy. Contrast-enhanced computed tomography indicated the ventriculoperitoneal shunt's pathway, running from the left ventricle, passing through the center of the breast mass, and leading into the abdominal cavity. Fears of shunt occlusion and potential infection due to the untreated breast cancer prompted surgical intervention after consultation with the neurosurgeon. The surgery involved rerouting the ventriculoperitoneal shunt from the left thoracoabdomen to the right, performing a left mastectomy, and removing the fistula in the abdominal wall to minimize the risk of cancer recurrence along the shunt pathway. Postoperative histopathological examination confirmed the initial diagnosis of invasive ductal carcinoma of a triple-negative subtype, with no malignancy detected in the removed abdominal wall fistula.
CONCLUSIONS: Taking into account prior cases of cancer metastasizing distantly due to ventriculoperitoneal shunts, our case emphasizes the necessity to consider additional preventative measures against cancer seeding. This approach is particularly significant when treating breast cancer that arises along the pathway of a ventriculoperitoneal shunt, apart from performing conventional breast cancer surgery.
Keywords: Breast Neoplasms, Mastectomy, Ventriculoperitoneal Shunt, Female, Humans, Aged, 80 and over, Neoplasm Recurrence, Local, Hydrocephalus, Carcinoma, Ductal
Background
Ventriculoperitoneal (VP) shunt placement is a common neurosurgical procedure for hydrocephalus. During the procedure, a tube is placed under the skin or in the breast. Therefore, if breast cancer develops in the breast on the side where the tube was implanted, the handling of the cancer is problematic; there have been few reports on this issue. In this report, we describe a case in which breast cancer occurred in the pathway of a VP shunt in which left mastectomy, VP shunt rerouting, and abdominal wall fistulectomy were performed.
Case Report
The patient was an 86-year-old woman who became aware of a mass in her left breast. She presented to our hospital for close examination and treatment. She had previously undergone VP shunt placement due to normal-pressure hydrocephalus. She also had Alzheimer disease. She required full assistance with her activities of daily living (ADL) and lived in an institutionalized care facility. She had not had regular cancer screenings, including breast cancer screenings. On palpation, an irregular, mobile mass with a maximum diameter of 36 mm was detected at 9 o’clock of the left breast (Figure 1). In addition, erythema was observed on the skin directly over the mass. Breast ultrasonography showed an irregular mass with indistinct borders, rough margins, and skin infiltration at 9 o’clock of the left breast. The maximum diameter was 36 mm and the nipple–tumor distance (NTD) was 19 mm. The VP shunt was seen in the center of the mass (Figure 2). There were no enlarged axillary lymph nodes. We performed core-needle biopsy (CNB) on the same day. The diagnosis was invasive ductal carcinoma (IDC) and the subtype was triple-negative. Blood examination showed CEA 4.1 ng/mL and CA15-3 11.7 U/mL; there were no other significant findings. Contrast-enhanced computed tomography (CT) showed that the VP shunt passed from the left ventricle to the temporal side of the left head and neck, through the center of the left mammary mass, and into the abdominal cavity via subcutaneous abdominal tissue (Figure 3). No obvious distant metastases were identified. Based on these results, the patient was diagnosed with breast cancer cT4bN0N0 (stage IIIB).
Preoperative chemotherapy was considered. However, when the patien’s ADL was taken into consideration, the decision was made not to perform chemotherapy. The patient was elderly and had low ADL, but after consultation with a neuro-surgeon, it was considered that the best treatment was surgery because if the breast cancer was left untreated, there was a possibility that the VP shunt would become obstructed and hydrocephalus symptoms would appear, and because the breast cancer was accompanied by skin invasion, ulcers would form as it progressed, which would cause pain. The surgical procedure consisted of left mastectomy, VP shunt rerouting, and abdominal wall fistulotomy. First, the neurosurgeon rerouted the VP shunt from the left thoracoabdomen to the right thoracoabdomen (Figure 4). Left mastectomy was followed by excision of the abdominal wall fistula to prevent recurrence in the shunt’s pathway. The peritoneal tube was removed with the tumor. Sentinel lymph node biopsy was not performed. Resection of the fistula in the pathway of the left thoracic shunt was difficult because the shunt ran just below the dermis (Figure 5). The postoperative period passed without problems and the patient was discharged from the hospital on postoperative day 7. Histopathology results showed invasive ductal carcinoma, the size was 30×21 mm, histological grade was grade III, estrogen receptor was negative, progesterone receptor was negative, human epidermal growth factor receptor 2 was negative, Ki-67 was high (>30%), and the subtype was triple-negative (Figure 6). The resected abdominal wall fistula showed no evidence of malignancy. Postoperative adjuvant chemotherapy was not performed in consideration of her ADL. The patient was alive and recurrence-free as of 4 months after surgery.
Discussion
VP shunt surgery is the standard procedure for hydrocephalus; a tube is placed in the abdominal cavity through the subcutaneous tissue of the breast or intramammary region. There have been few reports of breast cancer in the pathway of a VP shunt. There is currently no consensus on how to deal with shunt tubes when cancer occurs.
There are few reports of breast-related complications, except in cases of breast cancer occurring in the VP shunt pathway. Some case reports described the tip of the tube being deviated from the abdominal cavity into the breast, causing spinal fluid to accumulate in the breast [1–3], impaired breast development in adolescence after neonatal VP shunt creation [4], and VP shunt failure during breast surgery [5].
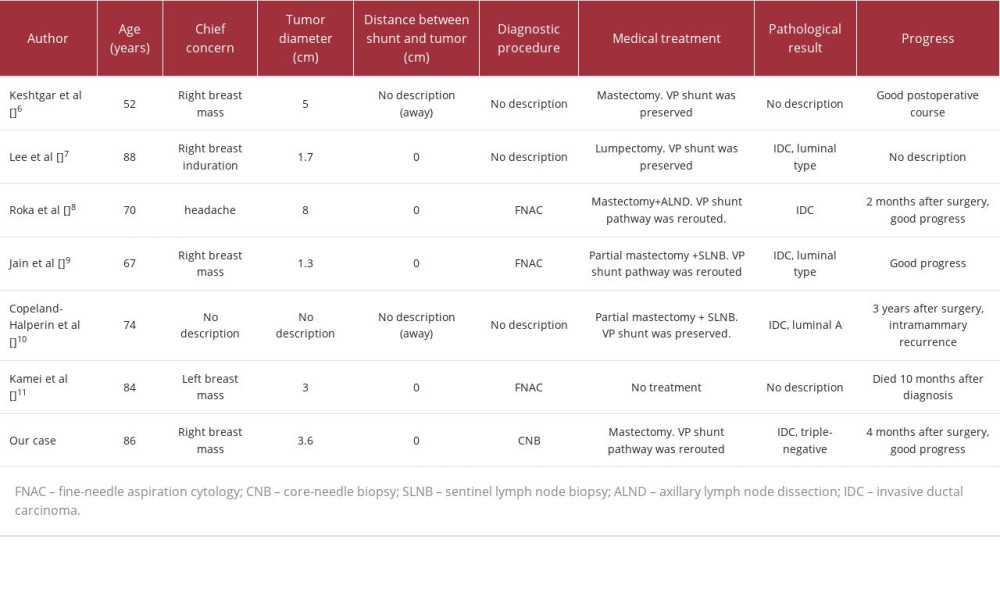
We compared 7 cases of breast cancer arising in the pathway of the VP shunt with our own case (Table 1) [6–11]. Patient age tended to be higher, ranging from 52 to 88 years. Most patients visited the hospital because of a breast mass. Tumor diameter varied from 1.3 cm to 8.0 cm. The pathohistological results were IDC in 6 cases and not described for 1 case. The subtype was luminal type in 3 cases, triple-negative in 1 case, and not described for 3 cases. We investigated the relationship between the VP shunt tube, a foreign body, and breast cancer histological type or subtype. However, there were no consistent trends except for the presence of IDC, even in cases where the tumor involved the shunt tube. The VP shunt tube and the development of breast cancer were not considered to be related, and in this case, the breast cancer was thought to arise accidentally, developed near the VP shunt, and spread, involving the VP shunt tube. Diagnostic methods were fine-needle aspiration cytology in 3 cases, CNB in 1 case, and not described for 3 cases. In all cases, great care must be taken not to damage the shunt tube during the examination procedure.
The tube was preserved in 3 cases and the shunt’s route was changed in 3 cases. In 2 cases where the tube was preserved, the tube was separated from the breast cancer. When shunt-sparing surgery is chosen, it is necessary to accurately diagnose the extent of the tumor based on breast US, CT, magnetic resonance imaging, and other imaging and histopathological findings. It is also necessary to carefully assess whether tube-sparing surgery is feasible. In such cases, preoperative breast ultrasound should be used to check the tube’s trajectory. Care should be taken to ensure that the tube is not exposed or damaged intraoperatively.
Cases of recurrent malignant disease associated with VP shunt have been reported. A case was reported in which the patient underwent surgery due to recurrence in the abdominal wall along the VP shunt after surgery for ascending colon cancer [12]. In addition, cases that may have spread cancer cells due to VP shunts were reported, including skin metastasis of pancreatic cancer [13], intracerebral metastasis of ovarian cancer [14], and intraperitoneal metastasis of brain tumors [15]. As in these reports, VP shunt can cause distant metastasis; abdominal wall recurrence and peritoneal seeding are expected to be problematic in breast cancers arising in the VP shunt pathway. Therefore, treatment should include routine surgery for breast cancer with additional procedures to prevent cancer seeding by the surgeon and neurosurgeon in close cooperation with each other. In the present case, mastectomy, VP shunt rerouting, and abdominal wall fistula creation were performed.
Conclusions
Cases of distant metastasis of cancer caused by VP shunt have been reported. Considering these reports, treatment of breast cancer associated with the VP shunt pathway should include additional procedures to prevent cancer seeding, in addition to routine breast cancer surgery. In this case, we performed mastectomy, VP shunt rerouting, and abdominal wall fistulotomy.
Figures
References:
1.. Shafiee S, Nejat F, Raouf SM, Coiling and migration of peritoneal catheter into the breast: A very rare complication of ventriculoperitoneal shunt: Childs Nerv Syst, 2011; 27(9); 1499-501
2.. Agarwal R, Allen A, Green L, Ventriculoperitoneal shunt catheter migration in a patient with breast implants resulting in a peri-implant cerebrospinal fluid pseudocyst: Radiol Case Rep, 2019; 14(2); 221-25
3.. Torres AN, Barraguer EL, Salvador Sanz JF, Late complication of a ventriculoperitoneal shunt in a patient with mammary prosthesis: J Plast Reconstr Aesthet Surg, 2008; 61(2); 212-14
4.. Jakeman M, Jeevan R, Burn SC, Falder S, Neurosurgical transection of the breast: An unexpected extracranial complication of ventriculoperitoneal shunt insertion: J Neurosurg Pediatr, 2017; 20(6); 517-20
5.. Zawadiuk LRR, Van Slyke AC, Macadam SA, Recurrent breast cerebrospinal fluid pseudocyst: A complication of ventriculoperitoneal shunt placement: Ann Plast Surg, 2019; 83(1); 108-11
6.. Keshtgar MR, Ahmed AR, Baum M, Ventriculo-peritoneal shunt and breast carcinoma: Ann R Coll Surg Engl, 2001; 83(4); 281-82
7.. Lee D, Cutler B, Roberts S, Manghisi S, Ma AM, Multi-centric breast cancer involving a ventriculoperitoneal shunt: Breast J, 2010; 16(6); 653-55
8.. Roka YB, Gupta R, Bajracharya A, Unusual cause for ventriculoperitoneal shunt failure: Carcinoma breast compressing distal catheter: Neurol India, 2010; 58(4); 662-64
9.. Jain YK, Kokan JS, An interesting case of screen-detected breast cancer encasing a ventriculoperitoneal shunt.: BMJ Case Rep., 2013; 2013 bcr2012007894
10.. Copeland-Halperin LR, Cohen RA, Recurrent breast cancer in a patient with a ventriculoperitoneal shunt.: Case Rep Surg, 2015; 2015; 659395
11.. Kamei M, Kikuchi N, Ichimura H, A case of breast cancer involving a ventriculoperitoneal shunt: Surg Case Rep, 2016; 2(1); 8
12.. Kataoka M, Kondo H, Hirano Y, Resection of solitary abdominal wall metastasis of ascending colon cancer along the ventriculoperitoneal shunt: A case report: Int J Surg Case Rep, 2021; 82; 105869
13.. Nawashiro H, Otani N, Katoh H, Subcutaneous seeding of pancreatic carcinoma along a VP shunt catheter: Lancet Oncol, 2002; 3(11); 683
14.. Eralp Y, Saip P, Aydin Z, Leptomeningeal dissemination of ovarian carcinoma through a ventriculoperitoneal shunt: Gynecol Oncol, 2008; 108(1); 248-50
15.. Ingold B, Moschopulos M, Hutter G, Abdominal seeding of an atypical teratoid/rhabdoid tumor of the pineal gland along a ventriculoperitoneal shunt catheter: Acta Neuropathol, 2006; 111(1); 56-59
Figures
In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250