17 September 2023: Articles 
A Case of Acute-Onset Type 1 Diabetes Mellitus with Diabetic Ketoacidosis Triggered by COVID-19
Unusual clinical course
Mariko Zenri12ABCDE*, Mariko Higa1ABE, Kayoko Ikehara12AB, Takamasa Ichijo1ABC, Takahisa Hirose2EFDOI: 10.12659/AJCR.940986
Am J Case Rep 2023; 24:e940986
Abstract
BACKGROUND: It is well known that diabetes mellitus contributes to COVID-19 severity. Recently, there have been reports of an increase in the number of children with type 1 diabetes after the COVID-19 pandemic.
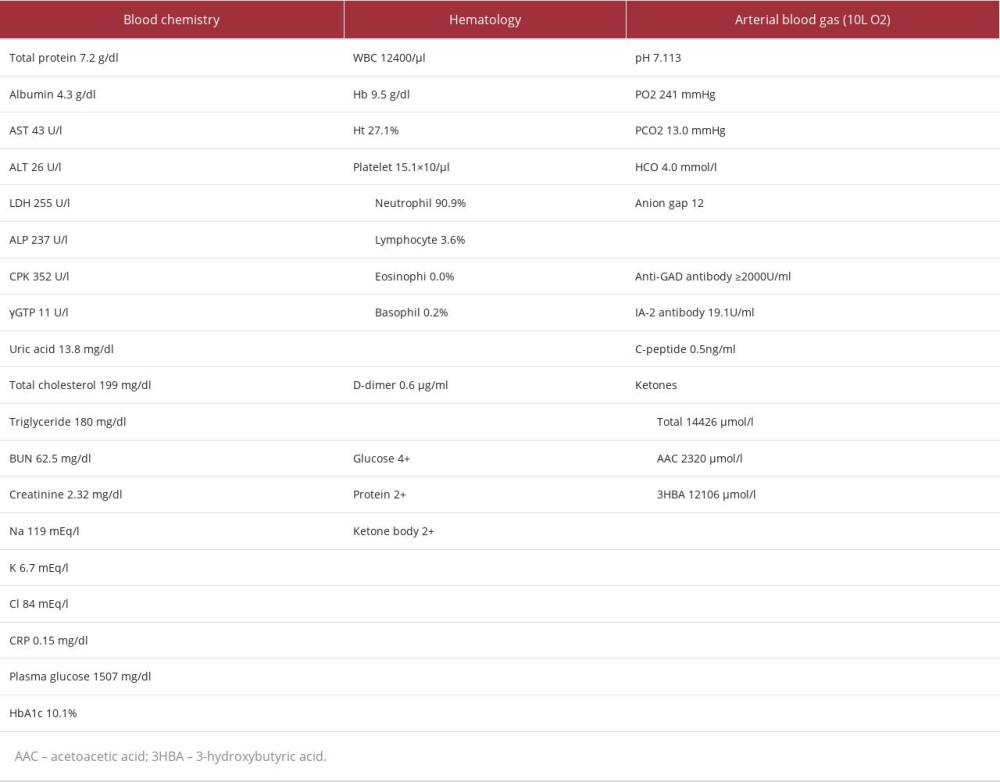
CASE REPORT: A 52-year-old woman presented to the Emergency Department with disturbance of consciousness, accompanied by a 1-day history of thirst, a fever of 38°C, and breathlessness. She had a positive coronavirus antigen test. Her initial vital signs assessment showed a heart rate of 120 beats per minute, blood pressure 90/50 mmHg, temperature 37.3°C, and respiratory rate 30 breaths/minute with an oxygen saturation of 100% with 10 L oxygen inhalation. Her initial laboratory test results showed a blood glucose level of 1507 mg/dl, HbA1c of 10.1%, ketone 2+, and blood gas pH 7.113. The patient was diagnosed with diabetic ketoacidosis (DKA). There were mild inflammatory findings with blood CRP 0.14 mg/dl and a white cell count of 12 400/μL, but no pneumonia on a chest CT scan. Therefore, the patient was diagnosed with COVID-19 and DKA. The patient was positive for anti-glutamic acid decarboxylase (anti-GAD antibody) and had markedly low levels 24-h urine C-peptide (CPR). She was diagnosed with acute-onset type 1 diabetes mellitus, as her blood examination showed a postprandial blood glucose level of 100 mg/dl and HbA1c of 5.7% 2 months before admission. After admission, fluid replacement and continuous intravenous insulin infusion therapy were started, and blood glucose and blood gas pH improved over 10 h.
CONCLUSIONS: There have been reports of cases of type 1 diabetes consequences of COVID-19, but the mechanism has not been elucidated.
Keywords: COVID-19, Diabetes Mellitus, Type 1, Diabetic Ketoacidosis, Child, Female, Humans, Middle Aged, COVID-19, Blood Glucose, glycated hemoglobin, Pandemics
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, Hubei, China in 2019 [1] and quickly spread worldwide. In March 2020, the World Health Organization (WHO) declared the novel coronavirus infection (COVID-19) a pandemic [2]. To date, a cumulative total of 676.02 million people have been infected worldwide, and the death toll has reached 6.87 million (https:www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---23-november-2021). COVID-19 is a disease that mainly causes respiratory symptoms and is fatal in severe cases; furthermore, diabetes and obesity are known relative risks for death, severe illness, and acute respiratory distress syndrome [3–5]. Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) are severe acute metabolic failures in diabetic patients, and there have been case reports of them occurring as complications at the onset of COVID-19 [6,7]. An analysis of patients with diabetes mellitus at the time of COVID-19 onset reported that 21.6% had known diabetes, 20.8% had previously complicated diabetes that led to the diagnosis for the first time, and 28.4% had newly developed diabetes due to infection or steroid hormone use [6]. Reports of cases in which type 1 diabetes developed simultaneously with the onset of COVID-19 have also been observed, although they are rare, and analyses and studies on the relationship between SARS-CoV-2 infection and the onset of type 1 diabetes, and its pathogenesis, have accumulated [8,9].
This report describes an adult case of acute-onset type 1 diabetes mellitus presenting DKA at the same time as the onset of COVID-19.
Case Report
A 52-year-old woman presented to our Emergency Department with disturbance of consciousness on August, 2020. She had thirst and fatigue 2 days prior to admission, and 1 day before admission she also experienced difficulty breathing and had a fever of 38°C; therefore, she visited the emergency room of Hospital A due to worsening dyspnea. A coronavirus antigen test was performed and was positive, but as there was no evidence of pneumonia on chest X-ray, the patient was judged to have a mild case of COVID-19 and was discharged with instructions to receive treatment at home. No blood collection or urinalysis was performed at that time. Immediately after returning home from Hospital A, she began to have impaired consciousness and was transported to our Emergency Department and was admitted to the Intensive Care Unit (ICU). She had been diagnosed with schizophrenia at the age of 25 and was on aripiprazole every 4 weeks by intramuscular injection. She had no family history of diabetes, no personal history of smoking or alcohol consumption, and no history of vaccination against COVID-19. She underwent a routine physical and blood examination 2 months prior to the onset of COVID-19, and no hyperglycemia was noted because her postprandial blood glucose level was 100 mg/dl and HbA1c level was 5.7%. Vital signs at the time of the Emergency Department visit included blood pressure 90/50 mmHg, pulse 120 beats/minutes, temperature 37.3°C, respiratory rate 30 breaths/minute, oxygen level 100% (with 10 L oxygen inhalation), and her level of consciousness was E3V5M6 on the GCS scale. Chest and abdominal findings were normal. The patient was not obese, with a height of 177 cm, weight of 53 kg, and BMI of 16.9 kg/m2.
Blood tests on admission showed a blood glucose level of 1507 mg/dl and HbA1c of 10.1%, indicating marked hyperglycemia (Table 1). She also had urine ketone 2+, strongly positive blood ketones, blood gas pH 7.113, HCO3− 4.0 mmol/L, and BE −24.8 mmol/L. Hence, she was diagnosed with DKA. Her blood level of CRP was low at 0.14 mg/dl, but procalcitonin (PCT) was high at 0.99 ng/ml, leukocytes were mildly elevated at 12 400/µL, and peripheral hemogram showed a low lymphocyte fraction of 3.6%. Blood ferritin level was mildly elevated at 225.9 ng/ml, while D-dimer was in the normal range at 0.6 μg/ml. A chest CT scan showed no evidence of pneumonia, but the patient required oxygen, leading to the diagnosis of moderate COVID-19 disease. Serum creatinine (S-Cr) and serum potassium (S-K) were also markedly elevated, and she was noted to have acute renal failure. Blood tests on admission were strongly positive for both anti-glutamic acid decarboxylase (anti- GAD antibody) and anti-insulinoma-associated antigen-2 (anti-IA-2 antibody), whereas blood C-peptide (CPR) was markedly low at 0.5 ng/ml. The CPR 24-h urine collection performed later showed that endogenous insulin secretory capacity was depleted at 6.3 μg/24 h, and a diagnosis of acute-onset type 1 diabetes mellitus with autoimmune etiology was made. Various virus antibody blood tests, other than SARS-CoV-2 virus, showed no significant increase in antibodies. The HLA genotypes were DRB1*0901-DQB1*0303, and she had a disease susceptibility haplotype for the development of Japanese type 1 diabetes. The search for diabetic complications revealed no retinopathy, nephropathy, or neuropathy.
After admission (Figure 1), continuous venous insulin infusion therapy (CVII) was started at 1 unit/hour, along with an infusion of 5 L of saline in 24 h. The blood glucose level gradually decreased from the start of treatment, and 10 h after the start of treatment, the blood glucose level was 247 mg/dl, and the blood gas pH was also improved to 7.463. Blood pressure gradually improved with only fluid replacement, and blood oxygenation improved to 98% SpO2 on room air. The patient’s level of consciousness improved as her general condition improved, and she started eating on the second hospital day, and her insulin therapy was changed to basal-bolus therapy.
She was started with multiple daily injection therapy at has 10 units of glargine once daily and 6–8 units of lispro before meals, her fasting blood glucose levels were 229–250 mg/dl on the second hospital day, and her blood glucose level improved gradually. On day 10 as an inpatient, the fasting blood glucose was 112 mg/dL, and the 2-h postprandial blood glucose was 142 mg/dL with 23 units/day of lispro insulin and 12 units/day of glargine insulin for a 1800-calorie diet, and she was discharged home on the same day. Her HbA1c level was 7.1% 1 month after admission. No antiviral medication was administered for COVID-19, but on the third hospital day, her temperature was 36.9°C, and the inflammatory findings in the blood improved.
Discussion
We report an adult case of acute-onset type 1 diabetes mellitus presenting with DKA at the same time as the onset of COVID-19. The patient had undergone a routine physical and blood examination 2 months before her COVID-19 and was not noted to have hyperglycemia, suggesting she was not markedly hyperglycemic then. On admission, anti-GAD and anti-IA-2 antibodies were markedly elevated, leading to a diagnosis of type 1 diabetes mellitus with an autoimmune etiology. The high HbA1c of 10.1% on admission suggested that she had been hyperglycemic for at least 1–2 months, and the SARSCoV-2 infection may have triggered marked hyperglycemia and the development of DKA. SARS-CoV-2 infection has previously been noted to cause hyperglycemia and DKA [6,10]. This is considered to be due to the strong inflammation caused by viral infection, as well as the cytokine storm, an abnormal response of the immune system, inducing insulin resistance [6]. Anti-GAD antibody is an indicator that reflects autoimmunity against pancreatic beta cells [11] and is useful in diagnosing type 1 diabetes. The patient had not only high anti-GAD antibody levels, but also positive IA-2 antibody, an islet autoanti-body, and depleted endogenous insulin secretory capacity at onset, as well as Japanese type 1 diabetes disease susceptibility HLA type [12], which were considered consistent with acute-onset type 1 diabetes. Despite this, it is possible that aripiprazole, which the patient was on for schizophrenia, contributed to the hyperglycemia [13] in this case.
There have been reports of an increase in the number of children with type 1 diabetes after the COVID-19 pandemic compared to pre-pandemic [9], and an increase in the incidence of diabetes in adults after SARS-CoV-2 infection [14]. It is well known that type 1 diabetes can develop after infection with viruses such as enteroviruses and Coxsackie A and B viruses, and endogenous insulin secretion is severely impaired as a result of islet destruction by the virus, leading to the development of type 1 diabetes [15]. Type 1 diabetes is classified as idiopathic or autoimmune according to the pathogenic mechanism and the presence or absence of islet autoantibodies [15]. This patient was diagnosed with autoimmune type 1 diabetes because anti-GAD and anti-IA-2 antibodies were positive. SARS-CoV-2 virus enters cells through binding of the viral spike protein to the host cell surface angiotensin-converting enzyme 2 (ACE2), which subsequently fuses and is taken up into the cell [16]. A previous study demonstrated that ACE2 receptors are expressed in exocrine glands and islets in normal pancreatic tissue, suggesting that SARS-Cov-2 can target and injure the pancreas, resulting in decreased endogenous insulin secretion [17]. A report of 64 patients with known type 1 diabetes mellitus who had COVID-19 noted that more than half of them became hyperglycemic during the course of the disease, and 45.5% developed DKA [18]. Although there have been reports of cases with elevated blood pancreatic enzymes in patients with COVID-19 due to the presence of ACE2 receptors in the pancreatic exocrine line [8], the blood amylase in our patient was in the normal range. On the other hand, there have been reports of autopsy findings confirming the presence of the virus in islets in patients who died of COVID-19 [17]. However, it is unclear whether the damage to the islets caused by SARSCoV-2 infection affected endogenous insulin secretion reduction in this case. The rate of pancreatic beta cell destruction is sometimes slow in adult immune-mediated type 1 diabetes [15]. Her endogenous insulin secretory capacity might have been depleted gradually.
SARS-CoV-2 infection is known to cause a cytokine storm from immune system overreaction, not just a viral infection [19]. In particular, diabetic patients have higher levels of inflammatory indices than non-diabetic patients, which may trigger an excessive immune response, resulting in a systemic cytokine storm and a tendency to develop severe disease [19]. However, it has been noted that improving blood glucose levels and insulin therapy itself can improve inflammatory responses and blood cytokine indices [19,20]. An analysis of 23 698 deaths during hospitalization with COVID-19 in England between March 11 and May 11, 2020 showed that in patients with type 1 diabetes, the odds ratio for risk of death adjusted for age, sex, poverty, region, and ethnicity was 3.51 (95% confidence interval: 3.16–3.90), whereas in patients with type 2 diabetes, it was 2.14 (95% confidence interval: 197–2.09) [21]. In particular, patients with DKA complications at the time of admission are reported to have longer hospital stays and higher mortality rates [21]. Our patient presented with DKA, but did not develop pneumonia or have a poor prognosis.
Conclusions
We managed a case of DKA at the onset of COVID-19, which led to the diagnosis of acute-onset type 1 diabetes mellitus. The high HbA1c on admission suggests that the hyperglycemia may have been present for 1–2 months prior; however, the SARS-CoV-2 infection may have increased insulin resistance and led to DKA. It is unclear how SARS-CoV-2 infection was involved in the development of acute-onset type 1 diabetes and DKA complications in this case. Accumulation of future cases is considered important.
References:
1.. Phelan A, Katz R, Gostin LO, The novel coronavirus originating in Wuhan, China: Challenges for global health governance.: JAMA., 2020; 323; 709-10
2.. Mahase E, Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction.: BMJ., 2020; 368 m1036
3.. Williamson EJ, Walker AJ, Bhaskaran K, Open SAFELY: Factors associated with COVID-19 death in 17 million patients.: Nature, 2020; 584; 430-36
4.. Kumar A, Arora A, Sharma P, Is diabetes mellitus associated with mortality and severity of COVID19? A meta-analysis.: Diabetes Metab Syndr, 2020; 14; 535-45
5.. Zhu L, She Z-G, Cheng X, Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes.: Cell Metabol, 2020; 31; 1068-77
6.. Khunti K, Prato SD, Mathieu C, COVID-19, hyperglycemia, and new-onset diabetes.: Diabetes Care, 2021; 44; 2645-55
7.. Kim Na-y, Ha E, Moon JS, Lee Y-H, Choi EY, Acute hyperglycemic crises with coronavirus disease-19: Case reports.: Diabetes Metab J., 2020; 44; 349-53
8.. Boddu SK, Aurangabadkar G, Kuchay MS, New onset diabetes, type 1 diabetes and COVID-19.: Diabetes Metab Syndr, 2020; 14; 2211-17
9.. Salmi H, Heinonen S, Hästbacka J, New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic.: Arch Dis Child, 2021; 107(2); 180-85
10.. Li J, Wang X, Chen J, COVID-19 infection may cause ketosis and ketoacidosis.: Diabetes Obes Metab, 2020; 22(10); 19135-41
11.. Regnell SE, Lernmark A, Early prediction of autoimmune (type 1) diabetes.: Diabetologia, 2017; 60; 1370-81
12.. Kawabata Y, Ikegami H, Awata T, Differential association of HLA with three subtypes of type 1 diabetes: Fulminant, slowly progressive and acute-onset.: Diabetologia, 2009; 52; 2513-21
13.. Campanella LM, Lartey R, Shin R, Severe hyperglycemic hyperosmolar nonketotic come in a nondiabetic patient receiving aripirazole.: Ann Emerg Med, 2009; 53; 264-66
14.. Gohil A, Malin S, Abulebda K, Hannon T, A complicated case of COVID-19 and hyperglycemic hyperosmolar syndrome in an adolescent male.: Horm Res Paediatr, 2021; 94; 71-74
15.. , Standards of medical care in diabetes-2022.: Diabetes Care, 2022; 45(Suppl. 1); S17-38
16.. Hoffmann M, Kleine-Weber H, Schroeder S, SARS-Cov-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor.: Cell, 2020; 181; 271-80
17.. Wu C-T, Lidsky PV, Xiao Y, SARS-Cov-2 infects human pancreatic β cells and elicits β cells impairment.: Cell Metab, 2021; 33; 1565-76.e5
18.. Ebekozien OA, Noor N, Gallagher MP, Aloso GT, Type 1 diabetes and COVID-19: Preliminary findings from a multicenter surveillance study in the U.S.: Diabetes Care., 2020; 43; e83-85
19.. Sardu C, D’Onofrio N, Balestrieri ML, Outcomes in patients with hyperglycemia affected by COVID-19: Can we do more on glycemic control?: Diabetes Care, 2020; 43; 1408-15
20.. Ghanim H, Lohano T, Korzeniewski K, Suppressive effect of insulin infusion on chemokines and chemokine receptors.: Diabetes Care, 2010; 33; 1103-8
21.. Barron E, Bakhai C, Kar P, Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study.: Lancet Diabetes Endocrinol, 2020; 8; 813-22
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250