20 October 2023: Articles 
Infection Presenting with Myalgia and Cellulitis
Unusual clinical course, Challenging differential diagnosis
Hitomi Shimada12AEF, Risa HirataDOI: 10.12659/AJCR.941777
Am J Case Rep 2023; 24:e941777
Abstract
BACKGROUND: Helicobacter cinaedi is a rare bacterium, accounting for only 0.2% of the positive isolates in blood cultures. Previous reports note that patients with H. cinaedi infection often have underlying diseases. H. cinaedi infection is diagnosed by blood culture. However, because of the slow growth of this bacterium in blood culture, the diagnosis can be missed.
CASE REPORT: A 78-year-old man gradually developed erythema and pain in his left arm, then left shoulder and both lower legs. The patient presented to our hospital on day 17. He was afebrile, but the examination was remarkable for tenderness in both gastrocnemius muscles and erythema from the distal left lower leg to the ankle. We suspected pyomyositis and cellulitis and started oral administration of amoxicillin-clavulanate. On day 22, H. cinaedi was detected in blood cultures. Based on these findings, we diagnosed pyogenic myositis and cellulitis caused by H. cinaedi bacteremia. On day 24, antibiotic therapy was changed to intravenous ampicillin, and symptoms improved. Additional examination did not reveal any underlying immunodeficiency disorder, such as malignancy or HIV infection.
CONCLUSIONS: H. cinaedi infection can occur in healthy patients. Myalgia can be caused by pyogenic myositis because of bacteremia. In cases of myalgia or cellulitis of unknown etiology, blood cultures can be useful when bacteremia is suspected; blood samples should be monitored over an extended period.
Keywords: Bacteremia, Blood Culture, Helicobacter cinaedi, Myalgia, Male, Humans, Aged, Cellulitis, HIV Infections, Erythema, myositis
Background
Case Report
A healthy 78-year-old man developed erythema and warmth in the left elbow to the left forearm and both distal lower legs on day 0. The patient had no history of pet ownership, trauma, or sexual activity. On day 1, he visited his primary care physician, and he had inflammation in the left elbow joint; aspiration of the joint revealed clear fluid. The patient was prescribed nonsteroidal anti-inflammatory drugs, which improved the erythema in the left forearm, although the symptoms in both distal lower legs persisted. The patient developed sharp and severe sharp pain in the left shoulder on day 6, and stabbing pain in the gastrocnemius muscles of both legs and the left lower leg on day 7. Finally, on day 17, he presented to our hospital. During the clinical course, the patient had no symptoms such as diarrhea or abdominal pain suggesting gastrointestinal infection.
On admission, his vital signs were body temperature, 35.5°C; blood pressure, 150/80 mmHg; heart rate, 102 beats/min; respiratory rate, 12 breaths/min; and percutaneous oxygen saturation, 98% (room air). His body mass index was 21.5 kg/m2. Physical examination revealed tenderness in the gastrocnemius muscles of both legs and erythema in the left lower leg to the ankle area, but there was no swelling or warmth. There were no other remarkable findings on physical examination of the limbs, and no rash was observed. Physical examination of the chest and abdomen were also unremarkable.
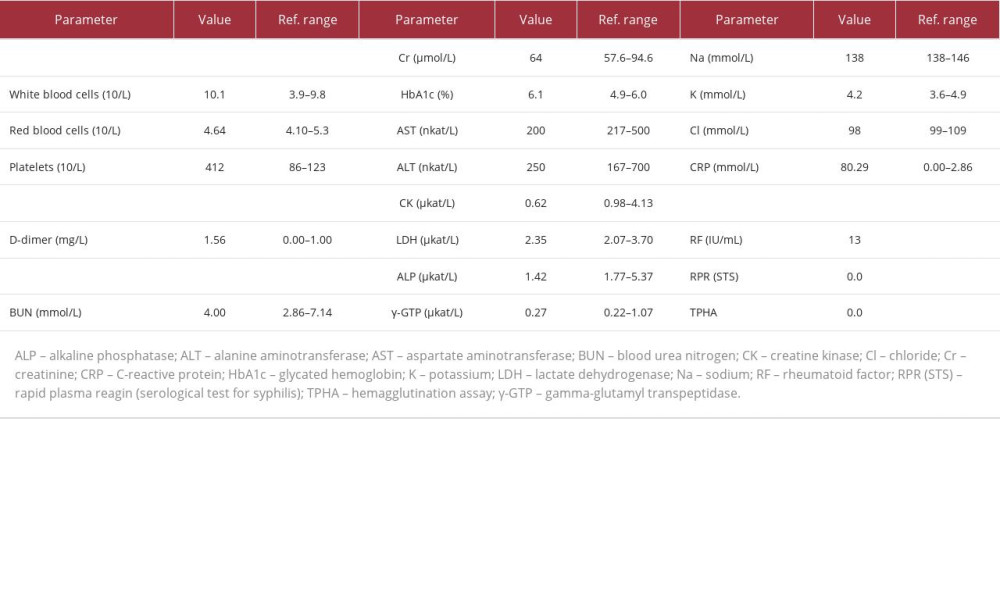
Blood tests showed a white blood cell count of 10.1×109/L, with neutrophils 69.6% (reference value: 41.8–73.8%), lymphocytes 21.5% (18.3–47.5%), and no abnormal cells in the peripheral blood. Hemoglobin level was 141 g/L, and platelet count was 412×109/L. Kidney and liver function were normal, with lactate dehydrogenase (LDH) level 2.35 µkat/L, C-reactive protein (CRP) level 80.29 mmol/L, ferritin level 0.611 nmol/L (0.04–0.562 nmol/L), soluble interleukin 2 receptor (sIL-2R) level 749 U/mL (121–613 U/mL), carcinoembryonic antigen (CEA) level 2.5 ng/mL (<5.0 ng/mL), and carbohydrate antigen 19-9 (CA19-9) level 11 U/mL (<37 U/mL). Syphilis and HIV antibodies were negative, and there was no complement deficiency. Anti-nuclear antibody, anti-aminoacyl tRNA synthetase (ARS) antibody, and anti-neutrophil cytoplasmic antibodies (ANCA) were negative as well (Table 1). At the first visit to our hospital on day 17, blood cultures using the BACTEC system were obtained. Urine tests were normal. Contrast-enhanced computed tomography of the chest, abdomen, and lower limbs revealed soft tissue swelling and subcutaneous fat tissue opacity in the left lower leg, extending to the ankle (Figure 1), but neither malignancy nor deep venous thrombosis was found.
Initially, cellulitis in the left lower leg was suspected; however, we also observed tenderness in the right lower leg, without any skin findings, which could not be fully explained by cellulitis alone. The patient had no conditions that could have caused muscle pain. For the differential diagnosis of diseases with muscle pain and an inflammatory reaction, we considered bacteremia, dermatomyositis, polymyositis, other myositis related to collagen disease, and pyogenic myositis caused by bacteria [8]. The anti-nuclear antibody, ARS antibody, and ANCA were negative, and the clinical course also ruled out myositis related to collagen disease.
By day 17, we had failed to make a definitive diagnosis, and so oral administration of clavulanate potassium-amoxicillin was started for cellulitis of the left lower leg. On day 22, the fifth day of cultivation,
After changing the antibiotics, the pain in both lower legs and left shoulder improved. Transthoracic echocardiography on day 24 revealed no vegetation or valvular disease, and brain magnetic resonance image on day 29 showed no infectious aneurysms or acute cerebral embolism. Although the patient had bacteremia, no findings suggested infective endocarditis. No malignant tumors that could cause immunodeficiency were found in the upper and lower gastrointestinal endoscopy examinations on day 27 and day 28, respectively. Blood cultures became negative on day 27, and the patient was discharged on day 31. Oral antibiotic therapy with amoxicillin was continued in the outpatient clinic until day 56. During the 90-day follow-up period, there was neither recurrence of
Discussion
This patient presented with muscle pain and cellulitis. The typical symptoms of
This patient presented with limb pain and skin erythema, but the diagnosis could not be made until day 22, when blood cultures became positive. Previously, a case of
Conclusions
In conclusion, even non-immunocompromised patients can have
References:
1.. Fennel CL, Totten PA, Quinn TC, Characterization of Campylobacter-like organizms isolated from homosexual men: J Infact Dis, 1984; 149; 58-66
2.. Araoka H, Baba M, Okada C: Helicobacter, 2018; 23(1); 12458
3.. Araoka H, Baba M, Kimura M, Abe M: J Clin Microbiol, 2014; 52(5); 1519-22
4.. Matsumoto T, Goto M, Murakami H: J Clin Microbiol, 2007; 45(9); 2853-57
5.. Uwamino Y, Muranaka K, Hase R: Helicobacter, 2016; 21(1); 24-28
6.. Nishida R, Shimono N, Miyake N: Intern Med, 2017; 56(6); 725-28
7.. Shimizu S, Inokuma D, Watanabe M: Acta Derm Venereol, 2013; 93(2); 165-67
8.. Molina B, Pogossian A, De Moreuil C, Rouvière B, Le Berre R, [Infectious myositis.]: Rev Med Interne, 2020; 41(4); 241-49 [in French]
9.. Morel F, Brossier F, Mbadi A: Med Mal Infect, 2015; 45(1–2); 41-43
10.. Taniguchi T, Saeki Y, Okayama A: Microbiol Immunol, 2017; 61(2); 57-63
11.. Bikels J, Ben-Sira L, Kessler A, Wientroub S, Primary pyomyositis: J Bone Joint Surg Am, 2002; 84(12); 2277-86
12.. Kiran M, Mohamed S, Newton A, Pelvic pyomyositis in children: Changing trends in occurrence and management: Int Orthop, 2018; 42(5); 1143-47
13.. Crum-Cianflone NF, Bacterial, fungal, parasitic, and viral myositis: Clin Microbiol Rev, 2008; 21(3); 473-94
14.. Crum NF, Bacterial pyomyositis in the United States: Am J Med, 2004; 117(6); 420-28
15.. Lambregts MMC, Bernards AT, van der Beek MT, Time to positivity of blood cultures supports early re-evaluation of empiric broad-spectrum antimicrobial therapy: PLoS One, 2019; 14(1); e0208819
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250