07 November 2023: Articles 
Paraneoplastic Neuromyelitis Optica Spectrum Disorder: A Rare Case of Advanced Breast Cancer with Intractable Nausea and Vomiting
Challenging differential diagnosis, Rare coexistence of disease or pathology
Daisuke MiyagishimaDOI: 10.12659/AJCR.941808
Am J Case Rep 2023; 24:e941808
Abstract
BACKGROUND: Neuromyelitis optica spectrum disorder (NMOSD) is a demyelinating disease of the central nervous system that includes the triad of transverse myelitis, optic neuritis, and area postrema syndrome (APS), characterized by intractable nausea and vomiting. NMOSD can be part of a paraneoplastic syndrome and is associated with seropositivity to aquaporin-4 (AQP-4). We present a patient with uncontrollable nausea and vomiting who developed herpes zoster and acute myelitis and was finally diagnosed with paraneoplastic NMOSD due to breast cancer.
CASE REPORT: A 51-year-old woman was hospitalized due to 2 weeks of intractable nausea and vomiting. Although contrast-enhanced thoracoabdominal computed tomography (CT) on day 4 suggested breast cancer in her left breast, the etiology of her symptoms remained unknown. On day 13, she developed herpes zoster, followed by acute myelitis on day 25. Magnetic resonance imaging (MRI) showing longitudinal extensive transverse myelitis and an elevated serum AQP-4 antibody level led to the diagnosis of NMOSD. Brain MRI detected a small lesion in the dorsal medulla oblongata, which explained the preceding APS. After starting intravenous methylprednisolone pulse therapy, her nausea and vomiting rapidly subsided. Breast cancer was resected on day 63, and immunohistochemical staining revealed overexpression of AQP-4 in the tumor cells, suggesting paraneoplastic NMOSD.
CONCLUSIONS: This report has highlighted the presentation and diagnosis of NMOSD and supports the possibility that this can present as part of a paraneoplastic syndrome. In addition, diagnosis of NMOSD preceded by APS requires meticulous history taking and careful interpretation of MRI in the dorsal medulla oblongata.
Keywords: Area Postrema, Breast Neoplasms, Herpes Zoster, Nausea, Neuromyelitis Optica, Paraneoplastic Syndromes
Background
Neuromyelitis optica spectrum disorder (NMOSD), previously known as Devic disease or neuromyelitis optica, is a demyelinating disease of the central nervous system that includes the triad of transverse myelitis, optic neuritis, and area postrema syndrome (APS), characterized by intractable nausea and vomiting. However, when the disease initially presents with nonspecific symptoms, such as nausea and vomiting, the diagnosis of NMOSD is complicated and usually delayed [1]. After the discovery of a disease-specific antibody called aqua-porin-4 (AQP-4) antibody, this disease was recognized as an autoimmune disease triggered by the produced AQP-4 antibody [2]. Although most NMOSD cases occur in an idiopathic condition, some present as a paraneoplastic syndrome associated with seropositivity to AQP-4 [3].
Here, we present a patient with uncontrollable nausea and vomiting who developed herpes zoster and acute myelitis and was finally diagnosed with paraneoplastic NMOSD due to breast cancer.
Case Report
A 51-year-old woman with no remarkable medical history presented to an outpatient clinic with severe fatigue, nausea, and vomiting of a 2-week duration. She had received 2 vaccinations against SARS-CoV-2 one year earlier and denied any infectious disease, including COVID-19, prior to her visit. She described the feeling of a bowling ball pressing from her back and the sensation of something heavy existing in her stomach. Continuous nausea induced periodic vomiting without any gastric contents 7 to 8 times daily, which was followed by hic-cups continuing for approximately 4 h. Blood tests and upper gastrointestinal endoscopy revealed no remarkable abnormalities; therefore, she was referred to our Gastroenterological Department for further evaluation.
On physical examination, she appeared extremely exhausted; however, her vital signs were stable, and the examination was unremarkable. She denied headache, numbness, weakness, vision loss, and other neurological symptoms. The patient was admitted to the gastroenterological ward for intravenous hydration therapy and further workup. Initial laboratory test results were within normal limits, except for mild hyponatremia (sodium 133 mEq/L; reference range, 138–145 mEq/L). Extensive blood examination revealed an elevated cortisol level, normal adrenocorticotropic hormone level, and elevated ketone levels (Table 1), reflecting the fasting state of the patient and excluding adrenal insufficiency and cyclic vomiting syndrome. While abdominal ultrasonography showed no abnormalities, contrast-enhanced thoracoabdominal computed tomography (CT) on day 4 of admission incidentally detected multiple enhanced nodules in the superior external quadrant of the left breast (Figure 1). Although these masses raised the possibility of malignancy, further investigation was postponed as they were not considered to be associated with her urgent symptoms. Nausea and vomiting persisted despite inhospital antiemetic treatment for 1 week. Brain magnetic resonance imaging (MRI) was performed on day 8 of admission to rule out cerebrovascular disease, revealing no abnormalities at the time of image acquisition.
On day 10 of hospitalization, she reported a stabbing sensation in the left leg, which was followed by the appearance of scattered erythema with small blisters along the L4–L5 dermatomes of the left leg (Figure 2). Herpes zoster was diagnosed, based on the clinical presentation, including typical prodromal pain and the unilateral dermatomal distribution of vesicular eruption. Treatment was initiated with amenamevir (400 mg once daily) for 7 days. The skin eruption gradually healed, whereas mild pain in the left leg persisted. Eleven days after the onset of herpes zoster, she was referred to a psychiatrist and diagnosed with somatic symptom disorder. She was started on sertraline hydrochloride at 25 mg/day.
On day 25 of admission, which was 12 days after the diagnosis of herpes zoster, the patient suddenly complained of weakness and numbness in the left leg and difficulty with urination. Neurological examination revealed mild weakness (Medical Research Council grade 3/5), numbness, and brisk reflexes in the lower left limb. She did not exhibit signs indicating cranial nerve involvement or cerebellar dysfunction. Spinal cord MRI revealed a longitudinal T2 hyperintense lesion, mainly in the gray matter extending from Th5 to the medullary cone (Figure 3), which was consistent with longitudinal extensive transverse myelitis. Lumbar puncture revealed mild pleocytosis (38 cells/μL; lymphocytes, 92.7%), with an elevated protein level (95 mg/dL). Polymerase chain reaction for varicella zoster virus DNA in the cerebrospinal fluid was negative. Over the next 2 days, her neurological symptoms progressively worsened, resulting in paraparesis and numbness in both legs. Based on a provisional diagnosis of postherpetic myeloradiculopathy, intravenous methylprednisolone (1 g/day) for 3 days and intravenous acyclovir (400 mg 3 times/day) were initiated on day 30 of admission. Nausea and vomiting completely disappeared after the second cycle of 3-day methylpredniso-lone 1 week later, and progressive deterioration of neurological symptoms stopped. These treatments were followed by oral prednisolone at 40 mg/day, which was gradually tapered after the addition of azathioprine.
The combination of symptoms, including an episode of acute transverse myelitis and intractable nausea/vomiting, led to the consideration of NMOSD in the differential diagnosis. Thereafter, on day 37 of admission, anti-AQP-4 immunoglobulin G (IgG) using enzyme immunoassay was assessed and its positivity was confirmed (6.7 U/mL). On retrospective evaluation of the brain MRI obtained on day 8 of admission, a tiny linear hyperintense lesion was noted in the dorsal medulla oblongata, which anatomically corresponded to the area postrema on fluid-attenuated inversion recovery (FLAIR) sequence (Figure 4). Brain MRI obtained on day 39 of admission revealed hyperin-tense signals with blurred borders in the dorsal medulla oblongata on FLAIR images (Figure 5), suggesting that these areas were responsible for the intractable nausea and vomiting and that the patient had APS. There were no abnormalities in the optic nerve or other regions of the brain. In addition to AQP-4-IgG positivity, clinical presentations of acute transverse myelitis and APS led to the definitive diagnosis of NMOSD, based on the 2015 diagnostic criteria for AQP-4-IgG-positive NMOSD [4].
During these treatments and diagnostic evaluations, the patient also underwent ultrasonography-guided core needle biopsy of the hypoechoic tumor, measuring 14 × 12 mm in the left breast on day 25 of admission, which was the day she reported weakness and numbness in the left leg. Histopathologic examination revealed scirrhous-type invasive ductal carcinoma, which was estrogen receptor-positive, progesterone receptor-positive, and human epidermal growth factor receptor 2-negative; Ki-67 labeling index was 5% (Figure 6A). Immunohistochemical staining revealed abundant AQP-4 expression in the cytoplasm of tumor cells, whereas the normal breast parenchyma was negative for AQP-4 (Figure 6B). Based on these findings, the final diagnosis was paraneoplastic NMOSD triggered by AQP-4-expressing breast cancer. On day 63 of admission, she underwent total left mastectomy with lymph node dissection, which confirmed the diagnosis of poorly differentiated invasive ductal carcinoma (T3, N1a, M0, stage IIIA, nuclear grading 1, histological grading 1) according to the eighth edition of the American Joint Committee on Cancer staging manual [5]. At the time of discharge for rehabilitation care, she was unable to live without a wheelchair, although she did not have nausea and vomiting.
Discussion
The unusual clinical course of this patient clearly illustrates the diagnostic difficulties of this rare disease when patients have nonspecific symptoms, such as nausea and vomiting. In addition, because it can occur as a paraneoplastic syndrome, vigilance is required to search for associated neoplasms, especially in elderly patients.
NMOSD, previously termed as Devic disease, is an inflammatory disorder that mainly involves the optic nerve and spinal cord and is characterized by relapsing episodes of vision loss and paralysis. Our understanding of the pathophysiology underlying NMOSD has progressed since the discovery of AQP-4 IgG in 2004. NMOSD is now regarded as a distinct autoimmune disease induced by AQP-4 IgG [6]. AQP-4 is a water channel protein expressed in astrocytes in the central nervous system and abundantly distributed in the spinal cord gray matter and the periaqueductal and periventricular regions of the brain. AQP-4 IgG binds to AQP-4, activates the complement, and damages astrocytes in the central nervous system [1]. Although the trigger for autoimmunity remains unclear in most patients, in some cases NMOSD has been reported to be associated with infections, vaccinations, and neoplasms. Regarding the diagnosis of NMOSD, the revised international consensus diagnostic criteria for NMOSD require the presence of at least 1 of the core clinical characteristics, such as optic neuritis, acute myelitis, and APS, in addition to AQP-4-IgG seropositivity for the diagnosis of NMOSD with AQP-4 IgG [4].
Although nausea and vomiting are commonly encountered symptoms in various clinical settings at clinics or hospitals, the differential diagnosis includes a wide range of conditions in the absence of concomitant symptoms in patients with these nonspecific and low-yield symptoms. Finding additional clues for diagnosis requires the scrutiny of the patient’s medical history, drug use, alcohol consumption, concomitant symptoms (eg, headache, vertigo, chest pain, abdominal pain, and diarrhea), laboratory abnormalities (eg, inflammatory reaction, electrolyte abnormalities, and endocrine disturbances), and imaging findings (eg, ultrasonography, CT, MRI, and gastrointestinal endoscopy). In the present case, despite all efforts, including various clinical evaluations, the definitive diagnosis was not reached until the abrupt onset of acute myelitis, which was initially misdiagnosed as somatic symptom disorder. The vomiting center is located in the medullary floor of the fourth ventricle, including the area postrema, which abundantly expresses AQP-4. Therefore, AQP-4 antibodies target this area, triggering an inflammatory cascade and inducing intractable nausea, vomiting, and hiccups in patients with NMOSD. This condition, also known as APS, is included in the revised diagnostic criteria for NMOSD [4]. In a neuropathologic study, Popescu et al demonstrated that the area postrema was a selective target of disease processes in patients with NMOSD and suggested that patients with lesions in this area were more likely to present with nausea and vomiting [7]. Some case studies reported patients with unexplained nausea and vomiting who were later diagnosed with NMOSD [8–12]. The reported frequency of isolated APS at the time of NMOSD onset is 7.1% to 14% [1,10]. The reported median interval from the onset of vomiting to classical NMOSD symptoms (optic neuritis or transverse myelitis) ranges between 4 and 19 weeks [8,10]. Differential diagnosis is usually challenging in patients with nausea and vomiting as solitary symptoms. Shosha et al reported surprising and pessimistic data that 44 of 100 patients with NMOSD initially presented to gastroenterologists with nausea and vomiting and underwent extensive workup; however, none of these patients received the correct diagnosis and 20% of the patients were misdiagnosed [1]. A previous case series reported that 30% to 36% of patients with AQP-4-IgG-positive NMOSD experienced hiccups and vomiting [10,13]. The present patient also experienced periodic hiccup episodes lasting approximately 4 h after severe episodes of vomiting. Hiccups accompanied by unexplained nausea and vomiting can be a clue to the diagnosis of AQP-4-IgG-positive NMOSD. Some studies even recommend the evaluation of AQP-4 IgG in patients with unexplained and intractable vomiting [10]. For proper management of this rare and difficult-to-diagnose condition, more attention should be paid to detailed history-taking and careful interpretation of brain MRI, with a focus on potential involvement of the dorsal medulla oblongata.
Although NMOSD is primarily an idiopathic autoimmune disease, it also occurs as a paraneoplastic syndrome in 1.3% to 25% of patients [3,14–16]. Those with paraneoplastic NMOSD are generally older at disease onset (mean age, 50–55 years) [3,14–17], while the overall median age of onset for NMOSD ranges between 32 and 41 years. Paraneoplastic NMOSD is female-dominant (70.6%–91%), although the ratio is relatively lower than that for idiopathic NMOSD [3,13–14,17]. Onconeural antigens expressed on tumors are hypothesized to stimulate the host immune system to produce antibodies such as AQP-4 IgG, which cross-react with nervous tissue and induce damage in the central nervous system. Breast and lung cancers are the 2 most frequent tumors that trigger paraneoplastic NMOSD [3,14,16,17], and breast cancer accounts for 18.3% to 32% of all paraneoplastic NMODS cases [3,13,16,17]. Tumor expression of AQP-4 confirmed by immunohistochemical staining is necessary to demonstrate a causal link, and only these cases should be termed paraneoplastic NMOSD [16]. To the best of our knowledge, the expression of AQP-4 in breast cancer tissue was demonstrated in only 2 other cases. Armagan et al reported a 62-year-old woman who presented with fatigue, nausea, and vomiting and developed paraparesis 1 month later [18]. Invasive ductal carcinoma of the breast was diagnosed 2 months thereafter; AQP-4 IgG was detected in serum, and the expression of AQP-4 was confirmed in tumor samples. Carrillo et al described a 32-year-old woman who developed tetraparesis several days after biopsy collection from breast cancer [19]. The authors clearly demonstrated the presence of AQP-4 IgG in serum and the expression of AQP-4 in tumor tissue and indicated that this was likely the trigger of an autoimmune response. In 59.7% to 61.0% of all paraneoplastic NMOSD cases, NMOSD-related symptoms either preceded or occurred concurrently with cancer diagnosis, similar to that observed in the present case [3,16,19]. The most common initial presentation of paraneoplastic NMOSD was transverse myelitis; however, among patients with central nervous system symptoms, APS accounted for 83.3% [3]. Sepulveda et al reported that the most frequent presenting symptom in patients with paraneo-plastic NMOSD was severe nausea and vomiting, which were significantly more prevalent in these patients than in those without neoplasms (41.2% vs 6.6%, respectively) [14]. In addition, they identified several risk factors for paraneoplastic NMOSD, including presentation with APS (intractable nausea and vomiting), age >45 years, and male sex [14]. In patients over 45 years of age diagnosed with NMOSD and APS at presentation, careful cancer screening, particularly in breasts and lungs, should be considered.
There is no consensus on the treatment strategy for paraneoplastic NMOSD, and long-term outcomes remain unclear. Almost all previous studies on paraneoplastic NMOSD have recommended surgical treatment for the primary tumor and have reported positive outcomes after tumor resection [3,13,18,20]. This approach is understandable because tumor removal can reduce the burden of onconeural antigens. However, the duration and intensity of immunosuppressive therapies for paraneoplastic NMOSD remain a topic of debate.
In the present patient, preceding APS and immunohistochemical AQP-4 positivity in breast cancer tissue clearly indicated the neoplastic origin of NMOSD. However, the patient had a herpes zoster episode during the clinical course, which featured a unique presentation. Several case reports have illustrated the triggering role of varicella zoster virus (VZV) in the development of NMOSD. Antecedent viral infections were confirmed in 15% to 35% of NMOSD cases [21–23]. In addition to VZV, cytomegalovirus, herpes simplex virus, mumps virus, human immunodeficiency virus, hepatitis A virus, and dengue virus have also been reported as infectious pathogens preceding NMOSD. Two mechanisms are proposed to underlie the role of infections in NMOSD: bystander activation and molecular mimicry [21,22,24,25]. The bystander activation theory hypothesizes that inflammatory reaction damages the central nervous system tissue, inducing the release of AQP-4 and triggering the production of anti-AQP-4 antibodies. The molecular mimicry theory suggests that activated B cells produce antibodies that recognize both the microbial and self AQP-4 epitopes due to the antigenic similarity between the structural epitopes of the infectious agent and patient’s host proteins.
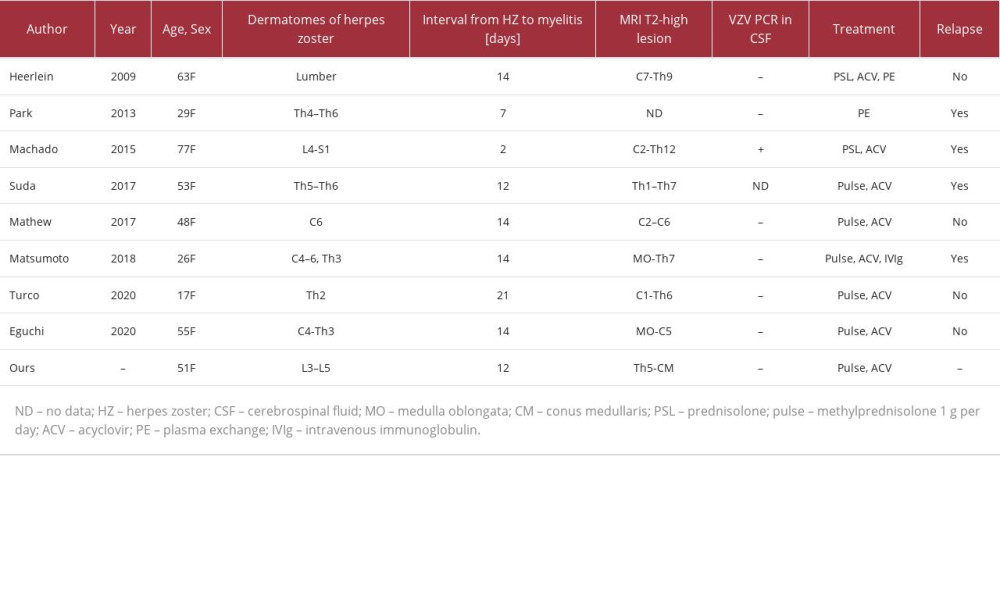
In our review of the literature, we identified 8 case reports with AQP-4-IgG-positive NMOSD preceded by herpes zoster (Table 2) [21,22,24–29]. The analysis of all 9 cases, including the present case, revealed that all patients were female, with a median age of 51 (17–77) years. The median interval between the onset of herpes zoster and acute myelitis was 14 (2–21) days. The dermatomes where the herpes zoster rash appeared did not always coincide with the spinal lesions on spinal cord MRI. Interestingly, all patients presented with an acute myelitis attack approximately 2 weeks after the onset of herpes zoster, whereas no patient had optic nerve symptoms. Turco et al reported a patient who was hospitalized due to unexplained vomiting 6 months before the appearance of vesicular rash of herpes zoster [29], suggesting that insidious APS due to NMOSD was present before the onset of herpes zoster, similar to that seen in the present case. The similarity of clinical presentation and close temporal relationship among these cases suggest a strong mutual relationship between herpes zoster and acute myelitis. It is possible that NMOSD alters cell-mediated immunity, resulting in the incidental reactivation of VZV; in such cases, herpes zoster can be considered as a sign heralding NMOSD. Alternatively, spontaneous VZV reactivation can affect the spinal cord and trigger acute myelitis with longitudinal extensive transverse myelitis in predisposed patients with AQP-4-IgG positivity. We agree with the opinion of Turco et al [23] that VZV can trigger longitudinal extensive transverse myelitis attacks because of the strict temporal relationship between VZV reactivation and myelitis attacks. We speculate that spinal damage caused by VZV reactivation exposes AQP-4 to circulating anti-AQP-4 antibodies, triggers the inflammatory cascade, and results in progressive myelitis with longitudinal extensive transverse myelitis. Although the exact interaction between VZV and NMOSD remains unclear, VZV is unlikely to be a direct cause of NMOSD but should be considered a modifying factor in the clinical course of NMOSD.
Conclusions
This report has described the presentation and diagnosis of NMOSD and demonstrated the possibility that this can present as part of a paraneoplastic syndrome. Since establishing the diagnosis of NMOSD that occurs with only nausea and vomiting is extremely difficult, physicians should be aware of the features of this rare disease and interpret MRI carefully in the dorsal medulla oblongata. In addition, attention should be paid to potentially coexisting neoplasms (ie, breast and lung cancer) after diagnosing this disease, especially in patients over 45 years of age.
Figures
References:
1.. Shosha E, Dubey D, Palace J, Area postrema syndrome: Frequency, criteria and severity in AQP4-IgG-positive NMOSD: Neurology, 2018; 91; e1642-51
2.. Abe Y, Yasui M, Aquaporin-4 in neuromyelitis optica spectrum disorders: A target of autoimmunity in the central nervous system: Biomolecules, 2022; 12(4); 591
3.. Shahmohammadi S, Doosti R, Shahmohammadi A, Neuromyelitis optica spectrum disorder (NMOSD) associated with cancer: A systematic review: Mult Scler Relat Disord, 2021; 56; 103227
4.. Wingerchuk DM, Banwell B, Bennett JL, International consensus diagnostic criteria for neuromyelitis optica spectrum disorders: Neurology, 2015; 85; 177-89
5.. Giuliano AE, Connolly JL, Edge SB, Breast cancer – major changes in the American Joint Committee on Cancer eighth edition cancer staging manual: Cancer J Clin, 2017; 67(4); 290-303
6.. Trebst C, Jarius S, Berthele A, Update on the diagnosis and treatment of neuromyelitis optica: Recommendations of the neuromyelitis optica study group (NEMOS): J Neurol, 2014; 261; 1-16
7.. Popescu BF, Lennon VA, Parisi JE, Neuromyelitis optica unique area postrema lesions: Nausea, vomiting, and pathogenic implications: Neurology, 2011; 76; 1229-37
8.. Apiwattanakul M, Popescu BF, Matiello M, Intractable vomiting as the initial presentation of neuromyelitis optica: Ann Neurol, 2010; 68; 757-61
9.. Enweluzo C, Yarra P, Neuromyelitis optica: An often forgotten cause of intractable nausea and vomiting: Case Rep Gastroenterol, 2013; 7; 281-86
10.. Iorio R, Lucchinetti CF, Lennon VA, Intractable nausea and vomiting from autoantibodies against a brain water channel: Clin Gastroenterol Hepatol, 2013; 11; 240-45
11.. Wang L, Su HJ, Qi JL, Intractable nausea and vomiting as an uncommon presentation in an anti-aquaporin 4-positive patient: J Int Med Res, 2018; 46; 3411-16
12.. Dandu V, Siddamreddy S, Meegada S, Isolated area postrema syndrome presenting as intractable nausea and vomiting: Cureus, 2020; 12; e7058
13.. Cai G, He D, Chu L, Paraneoplastic neuromyelitis optica spectrum disorders: Three new cases and a review of the literature: Int J Neurosci, 2016; 126; 660-68
14.. Sepúlveda M, Sola-Valls N, Escudero D, Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin-4 antibodies: Mult Scler, 2018; 24; 1753-59
15.. Virgilio E, Vecchio D, Vercellino M, Paraneoplastic neuromyelitis optica spectrum disorders: A case series: Neurol Sci, 2021; 42; 2519-22
16.. Apiraksattayaku N, Songwisit S, Owattanapanich W, AQP4-IgG-positive neuromyelitis optica spectrum disorder and temporally detected neoplasm: Case report and systematic review: Mult Scler Relat Disord, 2022; 68; 104212
17.. Ding M, Lang Y, Cui L, AQP4-IgG positive paraneoplastic NMOSD: A case report and review: Brain Behav, 2021; 11; e2282
18.. Armağan H, Tüzün E, Içöz S, Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: Favorable response to cancer treatment: J Spinal Cord Med, 2012; 35; 267-69
19.. Carrillo P, Gorría T, Santana D, Aquaporin-4-positive triple-negative breast cancer presenting with paraneoplastic neuromyelitis optica spectrum disorder: Biomed Hub, 2022; 7; 11-16
20.. Liao W, Li C, Tang Y, Aquaporin-4 antibody positive short transverse myelitis associated with breast cancer: Mult Scler Relat Disord, 2019; 30; 119-22
21.. Heerlein K, Jarius S, Jacobi C, Aquarin-4 antibody positive longitudinally extensive transverse myelitis following varicella zoster infection: J Neurol Sci, 2009; 276; 184-86
22.. Park JS, Hwang SJ, Shin JH, Kim DS, A recurrent longitudinally extensive transverse myelitis with aquaporin-4 (AQP4) antibody after herpes zoster: J Neurol Sci, 2013; 334; 69-71
23.. Turco EC, Curti E, Pisani F, Granella F, Herpes zoster preceding neuromyelitis optica spectrum disorder: Casual or causal relationship? A systematic literature review: J Neurovirol, 2022; 28; 201-7
24.. Machado C, Amorim J, Rocha J, Neuromyelitis optica spectrum disorder and varicella-zoster infection: J Neurol Sci, 2015; 358; 520-21
25.. Eguchi H, Takeshige H, Nakajima S, Herpes zoster radiculomyelitis with aquaporin-4 antibodies: A case report and literature review: Front Neurol, 2020; 11; 585303
26.. Suda M, Tsutsumiuchi M, Uesaka Y, Hayashi N, [A case of anti aquaporin-4 antibody positive myelitis with hyperhidrosis, following herpes zoster.]: Rinsho Shinkeigaku, 2017; 57; 26-28 [in Japanese]
27.. Mathew T, Thomas K, Shivde S, Post herpes zoster infection neuromyelitis optica spectrum disorder: Mult Scler Relat Disord, 2017; 18; 93-94
28.. Matsumoto Y, Tsuchiya M, Norshalena S, Severe aquaporin 4-IgG-positive neuromyelitis optica with disseminated herpes zoster in a pregnant woman successfully treated with intravenous immunoglobulin: Mult Scler J Exp Transl Clin, 2018; 4; 2055217318758119
29.. Turco EC, Curti E, Maffini V, Neuromyelitis optica spectrum disorder attack triggered by herpes zoster infection: Mult Scler Int, 2020; 2020; 6151258
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250