13 April 2024: Articles 
Successful Superficial Blood Sampling to Localize a Fibroblast Growth Factor-23-Producing Tumor
Challenging differential diagnosis, Unusual setting of medical care, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Junjiro Rikitake1ABCDEF, Kenji AshidaDOI: 10.12659/AJCR.943152
Am J Case Rep 2024; 25:e943152
Abstract
BACKGROUND: Tumor-induced osteomalacia (TIO) is a paraneoplastic syndrome caused by aberrant fibroblast growth factor-23 (FGF-23)-producing tumors. Early surgical resection is the optimal strategy for preventing TIO progression. Thus, tumor localization is a priority for successful treatment. A simple and safe examination method to identify functional endocrine tumors is essential to achieve better outcomes in patients with TIO.
CASE REPORT: A 64-year-old Japanese man with recurrent fractures, hypophosphatemia, and elevated alkaline phosphatase and FGF-23 levels (109 pg/mL) was admitted to our university hospital and was diagnosed with FGF23-related hypophosphatemic osteomalacia. Notably, the superficial dorsal vein in the patient’s left foot exhibited a high FGF-23 level (7510 pg/mL). Octreotide and ¹⁸F-fluorodeoxyglucose (FDG) scintigraphy and systemic venous sampling revealed that the tumor in the third basal phalanx of the left foot was responsible for FGF-23 overproduction. Tumor resection resulted in a rapid decrease in serum FGF-23 levels and an increase in serum phosphorus levels.
CONCLUSIONS: Octreotide scintigraphy, FDG-positron emission tomography, and systemic venous sampling are the standard methods for localizing functional endocrine tumors. However, the limited availability and invasive nature of these examinations hinder effective treatment. Here, we highlight the importance of peripheral superficial blood sampling as an alternative to conventional systemic methods for confirming the presence of FGF-23-producing tumors. Clinicians should consider TIO as a potential cause of acquired hypophosphatemic osteomalacia. Furthermore, peripheral superficial vein blood sampling may be useful for confirming the localization of FGF-23-producing tumors.
Keywords: Oncogenic Osteomalacia, Fibroblast Growth Factor-23, Hypophosphatemia, Malignant Mesenchymal Tumor, Blood Specimen Collection
Introduction
Tumor-induced osteomalacia (TIO) is a paraneoplastic syndrome caused by the hypersecretion of fibroblast growth factor-23 (FGF-23), which results in excessive excretion of phosphate in the urine and the subsequent development of hypophosphatemia. It was estimated that 0.04–0.70 people per 100 000 per year develop TIO [1]. Functional endocrine tumors often exhibit FGF-23 overproduction, necessitating their resection [1]. However, tumor localization is often challenging [2]; in some cases, clinicians cannot resect the tumor because of localization failure. The successful identification and removal of FGF-23-secreting tumors lead to the rapid normalization of serum FGF-23 and phosphate levels, thereby correcting metabolic bone disorders. Therefore, the localization of the responsible tumor remains a major concern for clinicians [3].
Systematic exploration to localize the responsible tumor involves a range of diagnostic examinations, including computed tomography (CT), magnetic resonance imaging (MRI), and scintigraphy, including octreotide scintigraphy [1], 68Ga-DOTA-TOC/TATE positron emission tomography–CT (PET/CT) [1], 18F-fluorodeoxyglucose (FDG) scintigraphy [1], and systemic venous sampling [4]. The stepwise approach, recommended in the diagnostic algorithm [1], is essential to effectively conclude a firm diagnosis of TIO. Localizing the responsible tumors is a major issue in clinical practice [5], despite the un-complicated diagnosis of FGF-23-related hypophosphatemia.
Systemic venous sampling is useful for identifying the location of functional endocrine tumors [6]. This procedure has also been applied to diagnose other diseases, such as cavernous sinus sampling for Cushing’s disease [7], selective arterial secretagogue injection (SASI) test for functional pancreatic neuroendocrine tumors [8,9], adrenal vein sampling for primary aldosteronism [10], ovarian vein sampling for sex hormone-producing ovarian tumors [11–13], and parathyroid hormone sampling for hyperparathyroidism [14]. Despite the diagnostic usefulness of systemic venous sampling, the procedure requires specialized skills and equipment and carries the risk of critical adverse events [10,15,16]. Consequently, venous sampling procedures are conducted within specialized institutions and are reserved for specific patient cases.
However, a safer and simpler method for localizing the responsible tumor is required in clinical practice. We encountered a patient with hypophosphatemic osteomalacia, whose FGF-23-producing tumor was successfully detected in the left third toe. Our findings demonstrate the potential effectiveness of blood sampling from the superficial veins, although further studies are required to clarify its effectiveness.
Case Report
REVIEW OF PREVIOUS LITERATURE:
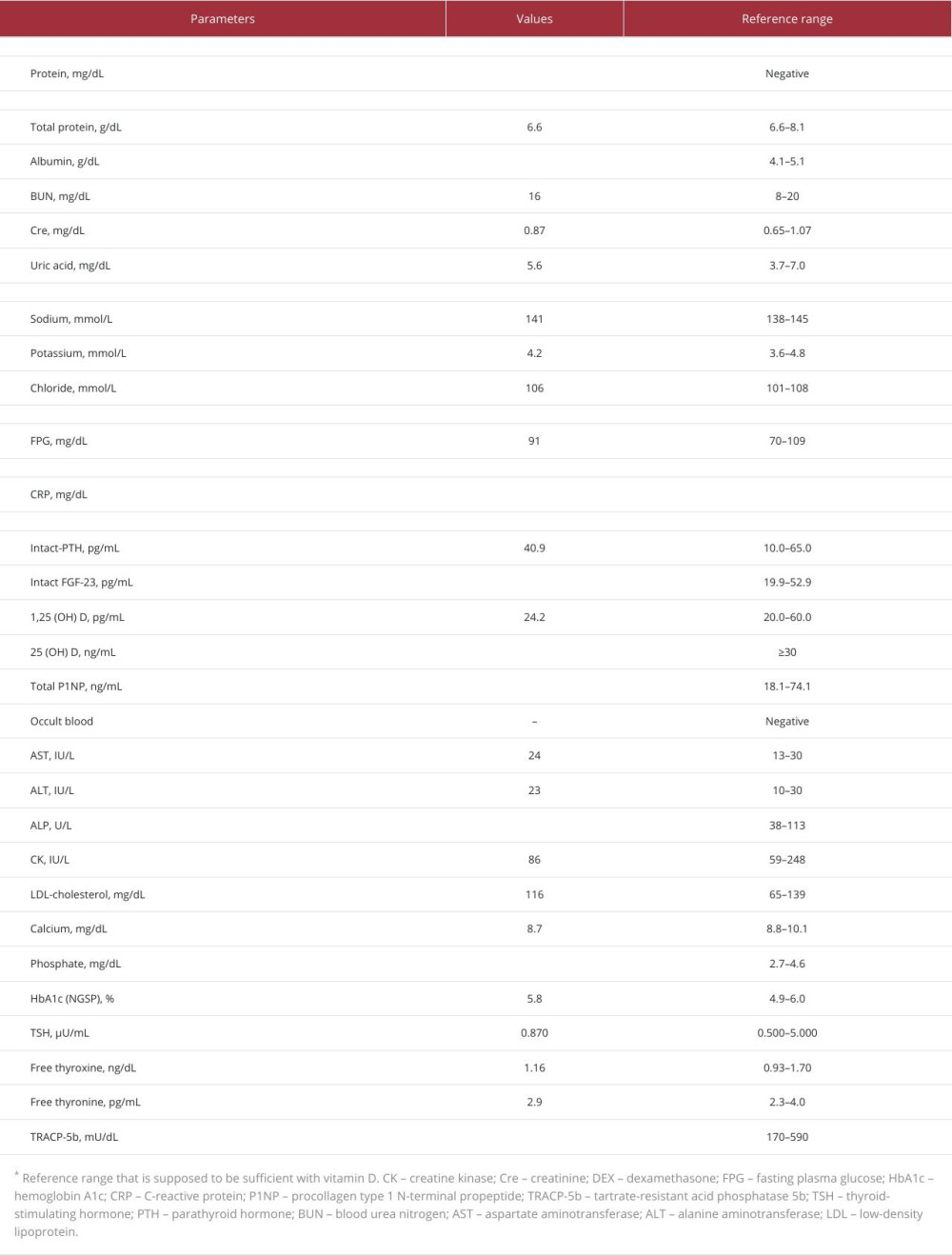
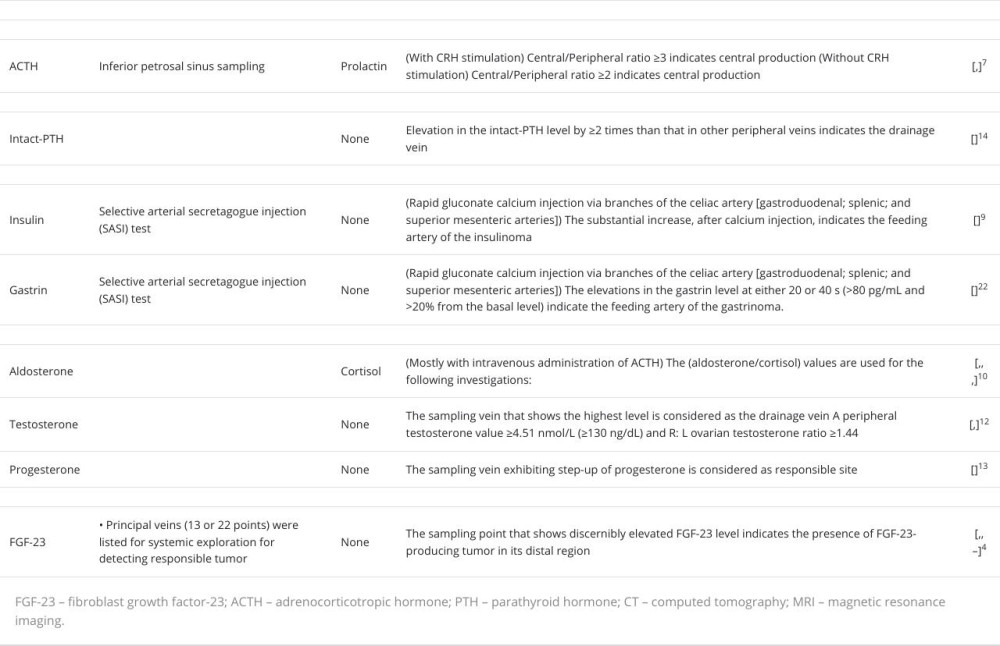
The current blood sampling methods are summarized in Table 2. Several hormones are targeted to identify functional endocrine tumors. These include adrenocorticotropic hormone (ACTH; pituitary gland), intact parathyroid hormone (parathyroid glands), insulin, gastrin (pancreas), aldosterone, testosterone, estradiol (adrenal and genital glands), and FGF-23 (bone or connective tissue).
Inferior petrosal sinus sampling has been performed to confirm the localization of ACTH-producing tumors or investigate the source of elevated ACTH levels and whether it originated from the pituitary gland [7,21]. This method is especially useful for the differential diagnosis of Cushing’s disease and ec-topic ACTH syndrome and for the localization of minimum pituitary adenomas.
Intact parathyroid hormone sampling is occasionally performed to identify pathological parathyroid adenomas, particularly in cases with discordant or negative imaging results. No definitive method has been established for this; however, sampling sites include the superior, middle, and inferior thyroid veins and the internal jugular and/or brachiocephalic veins [14].
SASI testing is performed to localize pancreatic neuroendocrine tumors, including insulinoma [9] and gastrinoma [22]. This method is useful in guiding surgical decisions, especially for small tumors that remain elusive on imaging examinations, such as CT, MRI, scintigraphy, and endoscopic ultrasound.
Adrenal vein sampling (AVS) is a popular method for confirming the laterality of aldosterone production [10,15,23].
AVS was performed to investigate aldosterone-producing tumors after confirming primary aldosteronism by the administration of captopril, furosemide in an upright position, and/ or saline infusion tests. Additionally, intravenous administration of ACTH was reported to enhance the accuracy of the results [24]. Eliminating bilateral aldosterone-producing disorders, bilateral adenomas, and idiopathic aldosteronism was a major concern in this method.
Venous testosterone sampling was performed to confirm the presence of functional endocrine tumors (usually adrenal or ovarian) responsible for virilization [12,25]. Additionally, this method is effective for identifying progesterone-producing tumors that remain undetected by imaging [13].
Systematic venous sampling has been proposed to confirm or detect FGF-23-producing tumors [4,26–29]. This examination would be beneficial for the surgical management and systemic screening of undetected tumors using imaging examinations such as CT, MRI, FDG-PET/CT, octreotide scintigraphy, or DOTA-TOC/TATE PET-CT.
Discussion
Here, we describe an effective method for FGF-23 sampling from the peripheral superficial vein to detect tumors responsible for TIO. Serum FGF-23 levels were notably higher in the dorsal foot vein compared to other peripheral veins using conventional sampling method. Our findings suggest that clinicians can safely and effectively apply this method to detect the responsible tumors in patients with TIO.
Peripheral superficial vein sampling for FGF-23 can effectively detect tumors responsible for TIO in approximately half of patients. The tumors were localized across various regions, with the highest frequency in the legs (42%), followed by the head and face (21%), hip and pelvic domains (12%), trunk (11%), and arms (9%) [5]. Thus, obtaining positive results for the detection of functional endocrine tumors using peripheral superficial vein blood sampling may occur in >50% of cases. When a tumor is detected using CT, MRI, or octreotide scintigraphy, this simple method can contribute to determining the location of FGF-23 production. Additionally, in cases where a tumor cannot be detected [27], superficial vein sampling from the peripheral legs and arms may still offer clues for clinicians to detect the tumor. Moreover, as exemplified in this case, distinguishing between fractures and tumors is imperative in the clinical assessment [3].
The FGF-23 level in the superficial dorsal foot vein was more than 7 times higher than that in the target peripheral veins, as confirmed by conventional systemic blood sampling. In clinical practice, an increase in target variables by >1.5–2.0 times compared to the reference site is typically considered significant [6]. This case demonstrated a 31-fold increase in serum FGF-23 levels in the dorsal vein compared to those in the peripheral forearm (negative site). Additionally, a higher level was noted compared with the levels observed in other areas that showed accumulation of both FDG and octreotide. Superficial vein sampling using the dorsal foot vein indicated a 7.3- to 8.0-fold surge in FGF-23 levels compared to those from the left anterior tibial vein, which can be drained from the FDG and octreotide-accumulated area using conventional systemic venous sampling. In this context, this case report reveals that sampling using the superficial vein can be more effective than conventional venous sampling, which carries the risk of complications [30]. Furthermore, this superficial method can detect step-ups, even in patients for whom positive results cannot be obtained using conventional systemic methods [31].
This study has some limitations. First, superficial venous sampling was effective in this particular case, but it may be ineffective for FGF-23-producing tumors localized in deeper regions. Second, clinicians should conduct the traditional venous sampling to localize the tumor, especially in cases of undetectable masses or unspecified step-ups in superficial sampling. Third, we did not perform venous sampling after surgery because FGF-23 levels decreased to the reference range after 3 h.
Conclusions
Here, we demonstrated the effectiveness of the peripheral superficial vein in detecting tumors responsible for TIO by measuring FGF-23 levels. Specific sampling methods for FGF-23-producing tumors allow clinicians to perform superficial venous sampling of FGF-23 as an initial screening procedure. In the present case, the FGF-23 levels were significantly higher in the dorsal foot vein than in specific peripheral veins, possibly because of drainage from the tumor site. These findings suggest that clinicians should consider peripheral superficial vein sampling as an alternative examination method for achieving successful outcomes in patients with TIO.
Figures
References:
1.. Minisola S, Fukumoto S, Xia W, Tumor-induced osteomalacia: A comprehensive review: Endocr Rev, 2023; 44; 323-53
2.. Feng J, Jiang Y, Wang O, The diagnostic dilemma of tumor induced osteomalacia: A retrospective analysis of 144 cases: Endocr J, 2017; 64; 675-83
3.. Dahir K, Zanchetta MB, Stanciu I, Diagnosis and management of tumor-induced osteomalacia: Perspectives from clinical experience: J Endocr Soc, 2021; 5; bvab099
4.. Takeuchi Y, Suzuki H, Ogura S, Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia: J Clin Endocrinol Metab, 2004; 89; 3979-82
5.. Jiang Y, Xia W, Xing X, Report of 39 cases and review of the literature: J Bone Miner Res, 2012; 27; 1967-75
6.. Andreopoulou P, Dumitrescu CE, Kelly MH, Selective venous catheterization for the localization of phosphaturic mesenchymal tumors: J Bone Miner Res, 2011; 26; 1295-302
7.. Fleseriu M, Auchus R, Bancos I, Consensus on diagnosis and management of Cushing’s disease: A guideline update: Lancet Diabetes Endocrinol, 2021; 9; 847-75
8.. Wada M, Komoto I, Doi R, Imamura M, Intravenous calcium injection test is a novel complementary procedure in differential diagnosis for gastrinoma: World J Surg, 2002; 26; 1291-96
9.. Thompson SM, Vella A, Thompson GB, Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis: J Clin Endocrinol Metab, 2015; 100; 4189-97
10.. Turcu AF, Auchus R, Approach to the patient with primary aldosteronism: Utility and limitations of adrenal vein sampling: J Clin Endocrinol Metab, 2021; 106; 1195-208
11.. Macut D, Ilić D, Mitrović Jovanović A, Bjekić-Macut J, Androgen-secreting ovarian tumors: Front Horm Res, 2019; 53; 100-7
12.. Nishiyama S, Hirota Y, Udagawa Y, Efficacy of selective venous catheterization in localizing a small androgen-producing tumor in ovary: Med Sci Monit, 2008; 14(2); CS9-12
13.. Duan L, Yang Y, Gu Y, The utility of adrenal and ovarian venous sampling in a progesterone-producing adrenal tumor and review of the literature: Endocrine, 2019; 66; 319-25
14.. Yang X, Chen X, Xu H, Selective venous sampling in primary hyper-parathyroidism caused by ectopic parathyroid gland: A case report and literature review: BMC Endocr Disord, 2023; 23; 141
15.. Dong H, Huang J, Zhang Y, Adrenal venous sampling via an antecubital approach in primary aldosteronism: A multicenter study: J Clin Endocrinol Metab, 2023; 109; e274-79
16.. Morita S, Yamamoto T, Kamoshida K, Safety and feasibility of unilateral double femoral venous access including double sheath insertion via a single-hole method for adrenal venous sampling: Jpn J Radiol, 2020; 38; 800-6
17.. Kato H, Miyazaki H, Kimura , Clinical performance of a new intact FGF23 immunoassay in healthy individuals and patients with chronic hypophosphatemia: Bone Rep, 2023; 18; 101659
18.. Fukumoto S, Ozono K, Michigami T, Pathogenesis and diagnostic criteria for rickets and osteomalacia – proposal by an expert panel supported by Ministry of Health, Labour and Welfare, Japan, The Japanese Society for Bone and Mineral Research and The Japan Endocrine Society: J Bone Miner Metab, 2015; 33; 467-73
19.. Colangelo L, Cipriani C, Pepe J, A challenging case of tumor-induced osteomalacia: Pathophysiological and clinical implications: Calcif Tissue Int, 2018; 103; 465-68
20.. Ni X, Zhang Z, Guan W, Shift in calcium from peripheral bone to axial bone after tumor resection in patients with tumor-induced osteomalacia: J Clin Endocrinol Metab, 2023; 108; e1365-73
21.. Ishida A, Asakuno K, Shiramizu H, Revalidation of inferior petrosal sinus sampling: The latest results from a single-center experience: Endocr J, 2021; 68; 1217-23
22.. Imamura M, Komoto I, Ota S, Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients: World J Gastroenterol, 2011; 17; 1343-53
23.. Umakoshi H, Tanase-Nakao K, Wada N, Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism: Clin Endocrinol, 2015; 83; 462-67
24.. Funder JW, Carey RM, Mantero F, The management of primary aldosteronism: Case detection, diagnosis, and treatment: An Endocrine Society clinical practice guideline: J Clin Endocrinol Metab, 2016; 101; 1889-916
25.. Levens ED, Whitcomb BW, Csokmay JM, Nieman LK, Selective venous sampling for androgen-producing ovarian pathology: Clin Endocrinol, 2009; 70; 606-14
26.. Kato H, Koga M, Kinoshita Y, Utility of multimodality approach including systemic FGF23 venous sampling in localizing phosphaturic mesenchymal tumors: J Endocr Soc, 2022; 7; bvac181
27.. Andreopoulou P, Dumitrescu CE, Kelly MH, Selective venous catheterization for the localization of phosphaturic mesenchymal tumors: J Bone Miner Res, 2011; 26; 1295-302
28.. Hidaka N, Koga M, Kimura S, Clinical challenges in diagnosis, tumor localization and treatment of tumor-induced osteomalacia: Outcome of a retrospective surveillance: J Bone Miner Res, 2022; 37; 1479-88
29.. Colangelo L, Sonato C, Cipriani C, Occipital bone and tumor-induced osteomalacia: A rare tumor site for an uncommon paraneoplastic syndrome: Arch Osteoporos, 2023; 18; 94
30.. McGee DC, Gould MK, Preventing complications of central venous catheterization: N Engl J Med, 2003; 3481123-33
31.. Oe Y, Kameda H, Nomoto H, Favorable effects of burosumab on tumor-induced osteomalacia caused by an undetectable tumor: Medicine (Baltimore), 2021; 100; e27895
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942921
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250