21 April 2024: Articles 
Successful Treatment of Lithium-Induced Nephrogenic Diabetes Insipidus with Celecoxib: A Promising Therapeutic Option
Unusual or unexpected effect of treatment
Ryunosuke Hashikawa1ABCDEF, Hiroyuki Yamada12ACDEF, Toshihito Fujii3DE, Shigeru Ohtsuru1EG*DOI: 10.12659/AJCR.943244
Am J Case Rep 2024; 25:e943244
Abstract
BACKGROUND: Nephrogenic diabetic insipidus (NDI) poses a challenge in clinical management, particularly when associated with lithium ingestion. Non-selective non-steroidal anti-inflammatory drugs (NSAIDs) have been widely used for the treatment of numerous diseases worldwide, including NDI. However, many studies have reported the diverse adverse effects of long-term use of non-selective NSAIDs. Celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is a better drug to relieve pain and inflammation in terms of long-term safety and efficacy than non-selective NSAIDs. Nevertheless, there are few reports describing the effectiveness of celecoxib in treating NDI.
CASE REPORT: We report a case of a 46-year-old woman with schizophrenia who presented with severe hypernatremia and refractory polyuria due to lithium-induced NDI. Cessation of lithium ingestion and traditional treatments, including trichlormethiazide and desmopressin, yielded minimal improvement in her hypernatremia and polyuria. Her sodium level needed to be strictly controlled with the infusion of dextrose 5% in water. Given the safety of celecoxib, we decided to initiate celecoxib as the treatment of lithium-induced NDI instead of indomethacin. Notably, the introduction of celecoxib led to a substantial and sustained amelioration of polyuria and hypernatremia without any celecoxib-associated adverse effects. Even after transfer to another hospital, stability in serum sodium levels persisted with celecoxib.
CONCLUSIONS: We presented a case of lithium-induced NDI successfully treated with celecoxib, a selective COX-2 inhibitor. To the best of our knowledge, this is the first reported case of successful treatment of lithium-induced NDI with celecoxib, and suggests celecoxib is a viable therapeutic option warranting further exploration. Physicians should consider its use when faced with the challenging management of lithium-induced NDI.
Keywords: celecoxib, Diabetes Insipidus, Nephrogenic, Lithium
Introduction
Nephrogenic diabetic insipidus (NDI) is an endocrine disease in which a decrease in the urine- concentrating ability is caused by resistance to arginine vasopressin (AVP) [1,2]. The most common cause of acquired NDI is chronic ingestion of lithium, which remains a mainstay of treatment for bipolar disorder [3,4]. Epidemiological studies reveal that NDI occurs in up to 40% of patients taking lithium [5]. Thiazide diuretics, amiloride, and indomethacin have been used to treat lithium-induced NDI. Nonetheless, some cases appeared to be irreversible despite cessation of lithium intake [6].
Non-steroidal anti-inflammatory drugs (NSAIDs) have been widely used for the treatment of numerous diseases worldwide. Among these medications, indomethacin, one of the cyclooxygenase (COX) non-selective NSAIDs, may have a better effect on NDI than other ones [5,7–9]. However, various complications may ensue with long-term use of COX non-selective NSAIDs. Many studies have reported its diverse adverse effects, such as gastroduodenal toxicity and bronchospasm [10,11]. COX-2 is an enzyme of prostaglandin-endoperoxide synthase identified in the early 1990s. Recent research has revealed the superiority of COX-2 selective NSAIDs over non-selective ones in terms of safety and efficacy [12–14]. These advantages have raised an exciting possibility to improve patient prognosis and quality of life in clinical practice.
Herein, we present a case of refractory lithium-induced NDI, successfully treated with celecoxib, a COX-2 selective NSAID.
Case Report
A 46-year-old woman with schizophrenia was transferred from a mental health hospital because of severe hypernatremia. She had been hospitalized in the previous hospital for treatment of psychotic exacerbation 5 days before the transfer. Her past medications included long-term lithium and levomepromazine, although the exact duration of lithium intake was unclear from her records. Her past medical history was insignificant other than schizophrenia. She never smoked or drank alcohol and had no history of substance abuse.
Upon examination, her temperature was 38.0°C, her pulse was 112/min and regular, her blood pressure was 130/89 mmHg, and her pulse oximetry on an O2 2L nasal cannula showed an oxygen saturation of 99%. She could not communicate due to confusion and agitation. Her blood laboratory test results showed a significantly elevated serum sodium level of >180 mmol/L, serum osmolarity of 369 mOsm/kg H2O, urine sodium level of 65 mmol/L, and urine osmolarity of 250 mOsm/kg H2O (Table 1).
Furthermore, she had polyuria of 3–4 liters per day, leading to prerenal kidney failure. She did not exhibit other electrolyte abnormalities. Her head and trunk computed tomography (CT) images revealed neither prominent intracranial nor internal organ diseases. These medical history and laboratory results raised the suspicion of lithium-induced nephrogenic diabetes insipidus. Thus, we discontinued her lithium treatment and administered trichlormethiazide and an infusion of dextrose 5% in water (D5W) to normalize her electrolyte balance. We controlled the amount of D5% infusion at around 1500 ml/ day, depending on her sodium level and urine volume. A fluid deprivation test was not conducted due to concerns about worsening her condition.
On the eighth day of follow-up, her sodium level improved, reaching 147 mEq/LL with the D5W infusion rate controlled (Figure 1), but her serum level rose rapidly after discontinuing the D5W infusion. It was quite challenging to stabilize serum sodium levels without the D5W infusion. Additionally, serum AVP concentration on the first day of admission showed 2.8 pg/mL (normal range, 0.5–5.0 pg/ml), indicating there was no positive feedback AVP secretion. Her brain magnetic resonance imaging (MRI) scan also revealed low intensity in the posterior lobe of the pituitary on T1-weighted MRI, which was consistent with central diabetes insipidus (Figure 2). To determine the cause of polyuria, we tried administering desmopressin nasal spray. Nevertheless, she remained polyuric, and her urine osmolarity was not elevated. Her sodium level also did not improve despite the AVP administration. These results confirmed that the cause of her polyuria and electrolyte disorder was lithium-induced NDI.
Trichlormethiazide and desmopressin did not improve her sodium level, so we initiated celecoxib as the treatment of NDI on day 30. Subsequently, her diuresis decreased to 1500–2500 ml/day, and intravenous D5% could be stopped. Her hyper-natremia also gradually and markedly resolved. Her Cre and eGFR showed no sign of deterioration and remained stable (Table 2). Although the urine test showed low osmolarity, the stable sodium level and the decreased urine output indicated good control and improvement of NDI.
On day 44, she was transferred to another hospital for long-term psychiatric treatment. A few months after the transfer, we have not received any reports of celecoxib-associated adverse effects.
Discussion
This female patient with schizophrenia suffered from refractory polyuria and hypernatremia after lithium ingestion. We administered trichlormethiazide and desmopressin to normalize her polyuria and electrolyte disorder due to lithium-induced NDI. However, these medications had only a minimal effect on her condition. Thus, she started receiving celecoxib on day 30, which dramatically and continuously improved her polyuria and hypernatremia. Even after her transfer to another hospital, her serum sodium level remained stable with celecoxib. To the best of our knowledge, this is the first reported case of lithium-induced NDI successfully treated with celecoxib.
Although indomethacin has a significant effect on decreasing urine output in NDI patients, its long-term administration clearly causes undesirable effects, such as gastrointestinal bleeding and kidney failure. Celecoxib, a selective COX-2 inhibitor, can relieve pain and inflammation with fewer adverse effects than classical non-selective NSAIDs. Its efficacy and safety have been demonstrated in several high-quality randomized controlled trials with adequate sample sizes [10,11,13,14] Thus, our case report’s highlight is that celecoxib can also be safe and effective for the polyuria of lithium-induced NDI as well as inflammatory diseases.
However, only 2 case reports have indicated the effectiveness of celecoxib in treating congenital NDI (Table 3). Tao et al reported celecoxib worked effectively for congenital NDI in combination with hydrochlorothiazide [15]. Soylu et al also have shown celecoxib was successful for treating congenital NDI [16]. These case reports strongly support our opinion that celecoxib is effective and safe for treating lithium-induced NDI.
Regarding the pathophysiology of NSAIDs for nephrogenic diabetes, COX-2 is predominantly produced in various kidney sites, including glomerular endothelium and medullary inter-stitial cells. Prostaglandin (PG) facilitates vasodilation of afferent arterioles and glomerulus and also inhibits the action of AVP of medullary interstitial cells [17]. Under the conditions of renal hypoperfusion, such as hypotension or chronic renal disease, PG synthesis increases to dilate renal afferent arterioles and glomerular vessels for adequate renal blood flow [18,19]. The COX inhibition by NSAIDs leads to decreased PG synthesis, resulting in renal vasoconstrictions and renal function decline. However, in patients without renal hypoperfusion, PG has little effect on renal hemodynamics [20,21]. Celecoxib, a selective COX-2 inhibitor, predominantly affects the medulla, inhibiting PG synthesis, increasing AVP action, and enhancing water absorption, as shown in our patient.
The limitation of this case report is that the low signal of the posterior pituitary lobe on T1-weighted MRI might be inconsistent with the diagnosis of lithium-induced NDI. However, the bright signal in this region is absent physiologically in some healthy individuals [22]. Additionally, our patient’s clinical course and endocrine hormone results strongly deny the possibility of central diabetes insipidus.
Conclusions
In this report, we presented a case of lithium-induced NDI successfully treated with celecoxib, a selective COX-2 inhibitor. Given the advantages of celecoxib, physicians should consider its use when faced with the challenging management of lithium-induced NDI.
Figures
References:
1.. Li JJ, Tan S, Kawashita T, Central diabetes insipidus in the background of lithium use: Consider central causes despite nephrogenic as the most common: Am J Case Rep, 2023; 24; e939034
2.. Robertson GL, Diabetes insipidus: Differential diagnosis and management: Best Pract Res Clin Endocrinol Metab, 2016; 30(2); 205-18
3.. Grünfeld JP, Rossier BC, Lithium nephrotoxicity revisited: Nat Rev Nephrol, 2009; 5(5); 270-76
4.. Malhi GS, Gessler D, Outhred T, The use of lithium for the treatment of bipolar disorder: Recommendations from clinical practice guidelines: J Affect Disord, 2017; 217; 266-80
5.. Stone KA, Lithium-induced nephrogenic diabetes insipidus: J Am Board Fam Pract, 1999; 12(1); 43-47
6.. Garofeanu CG, Weir M, Rosas-Arellano MP, Causes of reversible nephrogenic diabetes insipidus: A systematic review: Am J Kidney Dis, 2005; 45(4); 626-37
7.. Libber S, Harrison H, Spector D, Treatment of nephrogenic diabetes insipidus with prostaglandin synthesis inhibitors: J Pediatr, 1986; 108(2); 305-11
8.. Davis J, Desmond M, Berk M, Lithium and nephrotoxicity: A literature review of approaches to clinical management and risk stratification: BMC Nephrol, 2018; 19(1); 305
9.. Kim GH, Choi NW, Jung JY, Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2: Am J Physiol Renal Physiol, 2008; 294(4); F702-9
10.. Simon LS, Weaver AL, Graham DY, Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: A randomized controlled trial: Jama, 1999; 282(20); 1921-28
11.. Silverstein FE, Faich G, Goldstein JL, Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study: JAMA, 2000; 284(10); 1247-55
12.. Shin S, Safety of celecoxib versus traditional nonsteroidal anti-inflammatory drugs in older patients with arthritis: J Pain Res, 2018; 11; 3211-19
13.. Emery P, Zeidler H, Kvien TK, Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: Randomised double-blind comparison: Lancet, 1999; 354(9196); 2106-11
14.. Nissen SE, Yeomans ND, Solomon DH, Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis: N Engl J Med, 2016; 375(26); 2519-29
15.. Tao K, Awazu M, Honda M, An infant with congenital nephrogenic diabetes insipidus presenting with hypercalcemia and hyperphosphatemia: Endocrinol Diabetes Metab Case Rep, 2021 [Online ahead of print]
16.. Soylu A, Kasap B, Oğün N, Efficacy of COX-2 inhibitors in a case of congenital nephrogenic diabetes insipidus: Pediatr Nephrol, 2005; 20(12); 1814-17
17.. Berl T, Raz A, Wald H, Prostaglandin synthesis inhibition and the action of vasopressin: studies in man and rat: Am J Physiol, 1977; 232(6); F529-37
18.. Chou SY, Dahhan A, Porush JG, Renal actions of endothelin: Interaction with prostacyclin: Am J Physiol, 1990; 259(4 Pt 2); F645-52
19.. Oliver JA, Pinto J, Sciacca RR, Cannon PJ, Increased renal secretion of norepinephrine and prostaglandin E2 during sodium depletion in the dog: J Clin Invest, 1980; 66(4); 748-56
20.. Kim GH, Renal effects of prostaglandins and cyclooxygenase-2 inhibitors: Electrolyte Blood Press, 2008; 6(1); 35-41
21.. Weir M, Froch L, Weighing the renal effects of NSAIDs and COX-2 inhibitors: Clinical Dilemmas, 2000; 1; 3-12
22.. Colombo N, Berry I, Kucharczyk J, Posterior pituitary gland: Appearance on MR images in normal and pathologic states: Radiology, 1987; 165(2); 481-85
Figures
Tables
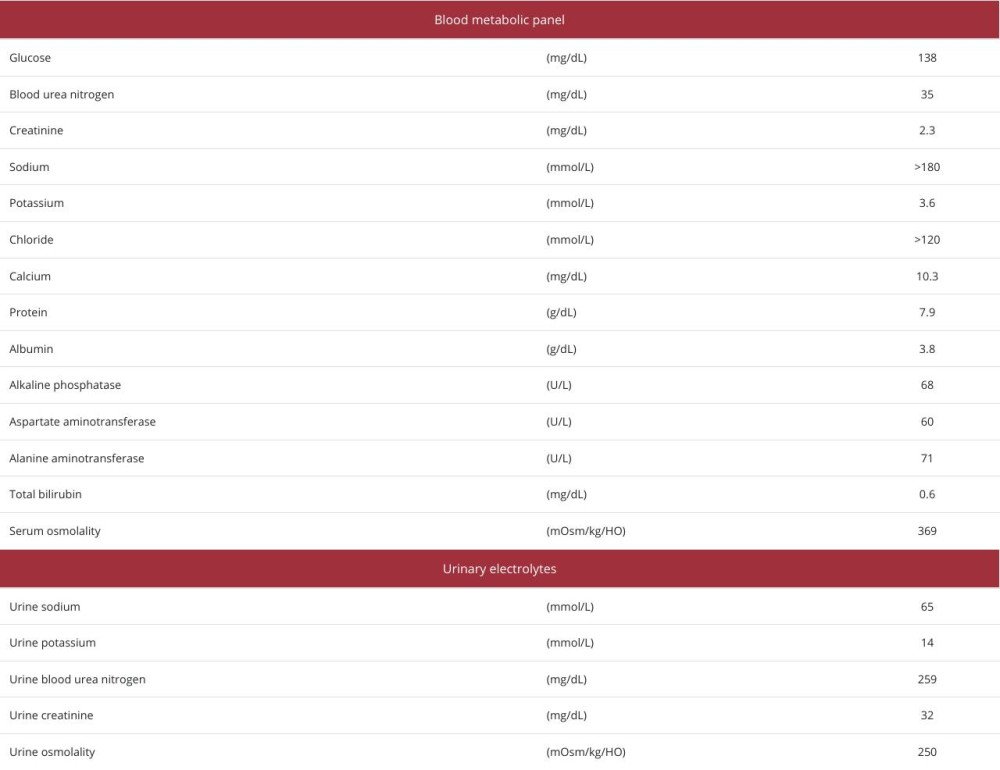 Table 1.. Blood and urine test results on admission.
Table 1.. Blood and urine test results on admission.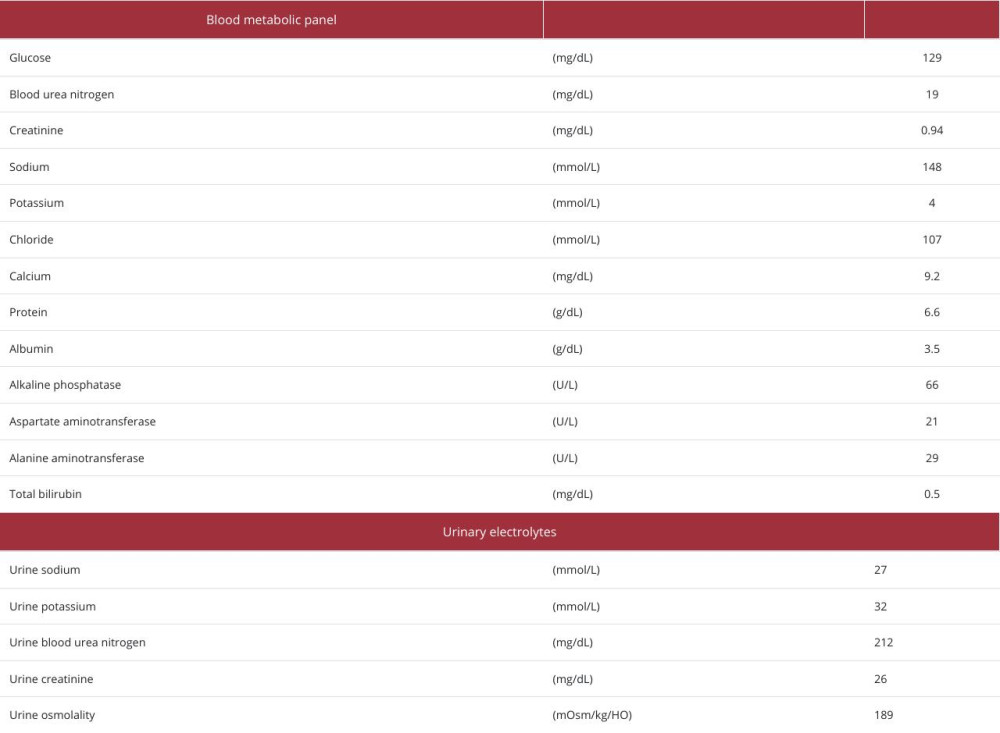 Table 2.. Blood and urine test results on day 40.
Table 2.. Blood and urine test results on day 40.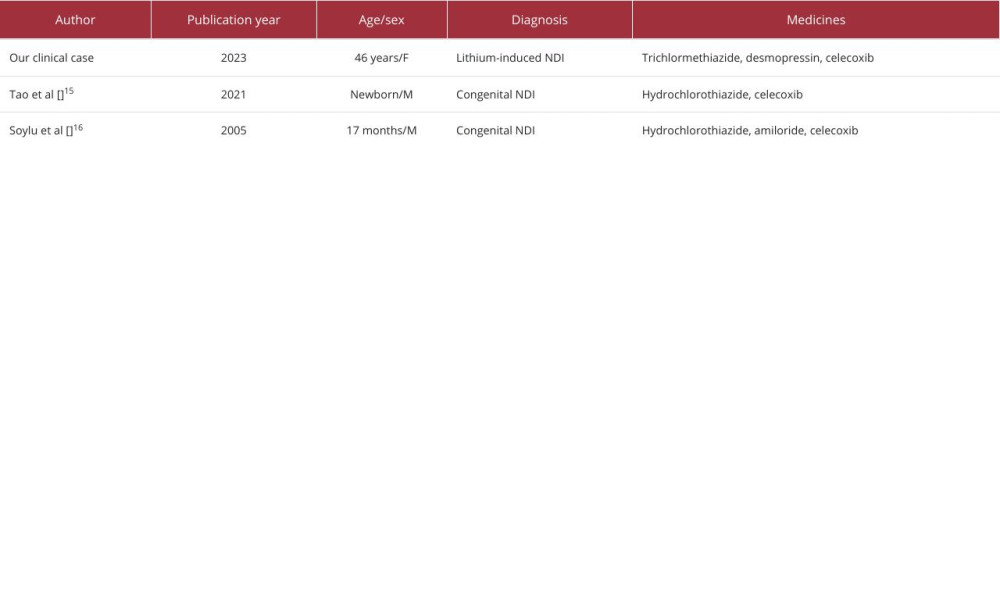 Table 3.. Published case reports of nephrogenic diabetic insipidus successfully treated with celecoxib.
Table 3.. Published case reports of nephrogenic diabetic insipidus successfully treated with celecoxib. Table 1.. Blood and urine test results on admission.
Table 1.. Blood and urine test results on admission. Table 2.. Blood and urine test results on day 40.
Table 2.. Blood and urine test results on day 40. Table 3.. Published case reports of nephrogenic diabetic insipidus successfully treated with celecoxib.
Table 3.. Published case reports of nephrogenic diabetic insipidus successfully treated with celecoxib. In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








