15 September 2020: Articles 
Ineffective Subtilisin/Kexin Type 9 (PCSK9) Inhibitors Monotherapy in Dyslipidemia with Low-Density Lipoprotein Cholesterol (LDL-C) Receptor Abnormalities: A Report of 2 Cases
Unusual or unexpected effect of treatment
Anthony Matta1234ABCDEF*, Dorota Taraszkiewicz123ABCDEF, Vanina Bongard123ABCDEF, Jean Ferrières123ABCDEFDOI: 10.12659/AJCR.923722
Am J Case Rep 2020; 21:e923722
Abstract
BACKGROUND: Real-life data on the efficacy of monotherapy with PCSK9 inhibitors are scarce. Most cohort studies have examined populations that are not severely dyslipidemic and are receiving combined therapy rather than monotherapy.
CASE REPORT: From a series of 167 alirocumab prescriptions, we present a case of complete nonresponse and one of low response to monotherapy with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in 2 patients with heterozygous familial hypercholesterolemia and abnormalities of the low-density lipoprotein cholesterol (LDL-C) receptor. In these cases, PCSK9 inhibitors were ineffective when used alone to reduce the LDL-C level, but the addition of statin led to a dramatic improvement.
CONCLUSIONS: As PCSK9 inhibitors become more commonly prescribed, more cases of nonresponse to PCSK9 inhibitors will be identified. Prospective studies are needed to investigate the efficacy of treatment with the monoclonal antibodies PCSK9 inhibitors in the context of LDL-C receptor abnormalities and to determine whether a genetic explanation exists for interindividual differences in response.
Keywords: Cardiovascular Abnormalities, Hypercholesterolemia, Lipid Regulating Agents, Cholesterol, LDL, dyslipidemias, Proprotein Convertase 9, Proprotein Convertases, Prospective Studies, Subtilisin
Background
Low-density lipoprotein cholesterol (LDL-C) is well known as a robust modifiable cardiovascular risk factor. Strong evidence from many studies supports that lowering LDL-C significantly slows the progression of atherosclerotic disease and reduces cardiovascular events, morbidity, and mortality. Indeed, European and American guidelines recommend intensive lowering of LDL-C, particularly in high-risk patients. Recently, pro-protein convertase subtilisin kexin 9 (PCSK9) inhibitors emerged as a promising medical therapy, offering a 60% reduction in the circulating level of LDL-C. Clinical trials of PCSK9 inhibitors have shown significant reductions for cardiovascular death, coronary revascularization, stroke, and myocardial infarction [1]. Because PCSK9 inhibitors have only been available for a short period of time, data on their application in real-world practice are limited. Moreover, reports of nonresponder cases are extremely rare in the literature [2]. Concerns have been raised regarding interindividual responses to PCSK9 inhibitors, and a genetically guided approach to their use has been suggested [3]. LDL-C apheresis is the ultimate solution for treatment of patients with serious dyslipidemias who do not respond to other therapy. A recently available cohort study showed results that were consistent with the clinical trials, with a good safety profile, even when PCSK9 inhibitors were used as monotherapy in completely statins-intolerant individuals [4]. To date, we have treated 167 patients with alirocumab in our lipid clinic. Among those treated with PCSK9 inhibitors alone for documented medical reasons, we report 2 cases of nonresponders to PCSK9 inhibitors alone. These cases were successfully managed by adding a simple statin dose, which led to dramatic changes in LDL-C levels.
Case Reports
CASE 1:
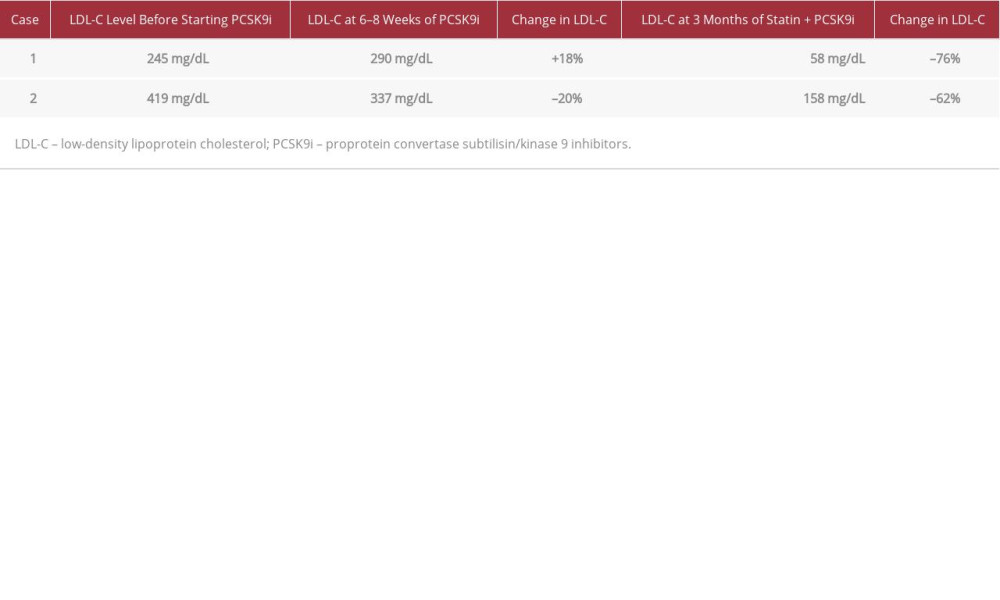
A 44-year-old man known to have familial hypercholesterolemia was referred to our lipid clinic for secondary prevention and LDL apheresis. His past medical history was significant for premature coronary artery disease with multiple stent angioplasties at 24 years of age. His medication list includes high-dose statin (atorvastatin, 80 mg), ezetimibe (10 mg), clopidogrel (75 mg), and nebivolol (5 mg). He reported severe myalgia and arthralgia leading to daily physical disability, and he was consequently considered intolerant to statin. Physical examination revealed tendon xanthomas, and blood tests showed total cholesterol (TC) of 324 mg/dL (normal range, 150–220 mg/dL), LDL-C of 228 mg/dL, high-density lipoprotein cholesterol (HDL-C) of 33 mg/dL (40–80 mg/dL), and triglycerides (TG) of 315 mg/dL (50–150 mg/dL). The patient’s genetic testing revealed a mutation in exon 4 for the LDL receptor; specifically, there was a missense mutation causing guanine replacement with adenine in position 682, resulting in glutamic acid replacing lysine at position 228. After 3 months, we halted his treatment regime and arranged for a blood test, which showed TC of 357 mg/dL (150–220 mg/dL), LDL-C of 245 mg/dL, HDL-C of 33 mg/dL (40–80 mg/dL), and TG of 395 mg/dL (50–150 mg/dL). According to the official recommendations in France, which restrict alirocumab reimbursement to those with an LDL-C level of more than 200 mg/dL for secondary prevention or more than 300 mg/dL for primary prevention in the setting of an experienced center in LDL-C apheresis, this patient was eligible for alirocumab reimbursement. We therefore decided to start a therapeutic approach with 150 mg of alirocumab by subcutaneous injection every 15 days. The first follow-up blood test result at 6-8 weeks, after 4 doses, showed TC of 374 mg/dL (50–150 mg/dL), LDL-C of 290 mg/dL (+18%), HDL-C of 35 mg/dL (40–80 mg/dL), and TG of 245 mg/dL (50–150 mg/dL). Incorrect injection technique and nonadherence to medication were omitted given that injections were provided by nursing staff. The patient was then diagnosed as a nonresponder to PCSK9 inhibitors, and in accord with the patient’s choice, we added a standard statin dose (atorvastatin 20 mg) and 10 mg of ezetimibe to prevent LDL-C apheresis. Three months later, blood tests revealed a dramatic improvement, with TC of 130 mg/dL, LDL of 58 mg/dL (76% reduction of baseline LDL-C), HDL of 37 mg/dL, and TG of 176 mg/dL. The patient tolerated this therapeutic regimen well.
CASE 2:
A 71-year-old woman known to have severe familial hyper-cholesterolemia and arterial hypertension was referred to our lipid clinic for primary prevention. Her past TC level had risen to 500 mg/dL, and she was considered statin intolerant at the time. Her blood test results performed under 40 mg of rosuvastatin and 10 mg of ezetimibe showed TC of 221 mg/dL (150–220 mg/dL), LDL-C of 133 mg/dL, HDL-C of 62 mg/dL (40–80 mg/dL), and TG of 132 mg/dL (50–150 mg/dL). We started by stopping the current medications and having genetic testing done, and a follow-up visit was scheduled for 1 month later. Genetic testing was positive for heterozygous deletion of exon 16 in the gene coding for the LDL receptor. A full resolution of symptoms (diffuse myalgia and daily exercise intolerance) was reported by the patient at the follow-up visit, but blood tests showed TC of 501 mg/dL (150–220 mg/dL), LDL-C of 419 mg/dL, HDL-C of 51 mg/dL (40–80 mg/dL), and TG of 155 mg/dL (50-150 mg/dL). We then began treatment with 150 mg of alirocumab by subcutaneous injection every 15 days, which was administered by nursing staff to avoid problems such as inappropriate injection and noncompliance with treatment. After 6 to 8 weeks, a blood test revealed a TC of 429 mg/dL (150–220 mg/dL), LDL-C of 337 mg/dL (20% reduction in LDL-C), HDL-C of 48 mg/dL (40–80 mg/dL), and TG of 218 mg/dL (50–150 mg/dL). Given this poor response to PCSK9 inhibitors and the patient’s preference to delay LDL apheresis, we added a low statin dose (10 mg of atorvastatin daily). Three months later, the patient reported mild, easily tolerable myalgia, and laboratory test results showed TC of 245 mg/dL (150–220 mg/dL), LDL-C of 158 mg/dL (62% reduction of baseline LDL-C level), HDL-C of 57 mg/dL (40–80 mg/dL), and TG of 151 mg/dL (50–150 mg/dL).
Discussion
Most real-world cohort studies do not include severely dyslipidemic populations being treated by combined therapy. The ODYSSEY trial showed that PCSK9 inhibitors are effective either when used alone or in combination with other lipid-lowering agents, while the FOURIER trial showed that PCSK9 inhibitors were effective on a background of statin therapy [3,5]. A 30 mg/dL drop in LDL-C, or a 59% reduction from LDL-C baseline, was revealed by the FOURIER trial when PCSK9 inhibitors were added to statin therapy [6]. A common indication for monotherapy with PCSK9 inhibitors is statin intolerance. In the 2 cases presented here, PCSK9 inhibitors were ineffective when used alone to reduce the LDL-C level in patients with heterozygous familial hypercholesterolemia and LDL-C receptor abnormalities. However, a dramatic improvement was achieved with the addition of statin (Table 1).
Available PCSK9 inhibitors (alirocumab and evolocumab) are monoclonal antibodies that bind to the PCSK9 protein responsible for regulating the expression of LDL-C receptors. Once PCSK9 binds to LDL-C receptors, it triggers lysosomal catabolism leading to an increased plasma level of circulating LDL-C. Inhibiting PCSK9 promotes the expression of LDL-C receptors on the extracellular surface, resulting in reduction of the circulating LDL-C [7]. In France, alirocumab is reimbursed only for patients with LDL-C >300 mg/dL being treated for primary prevention (case 2) or those with LDL-C >200 mg/dL being treated for secondary prevention (case 1) in the setting of an experienced LDL-C apheresis center within a university lipid clinic.
The 2019 European Society of Cardiology guidelines approved the clinical trials showing that PCSK9 inhibitors effectively reduce the level of LDL-C by 60% on average when used alone or in combination with other lipid-lowering agents, regardless of the previous therapy [1,8]. In addition, cohort studies have shown outcomes consistent with the findings from the clinical trials [4,9,10]. To the best of our knowledge, we are the first to report a case of complete nonresponse to PCSK9 inhibitors (case 1) without any observed reduction in circulating LDL-C. In fact, LDL-C rose by 18%. Saeed et al. [2] reported 2 cases of low response to PCSK9 inhibitors, which was defined by <30% reduction in LDL-C level. That report is consistent with our second reported case (i.e., 22% reduction in LDL-C). The short time that PCSK9 inhibitors have been available is a limiting factor with regard to the appearance of nonresponders. Over time, more nonresponder cases will emerge, especially when PCSK9 inhibitors are used as monotherapy.
The problem of failure to respond to PCSK9 inhibitors has been raised in literature. Several explanations have been proposed, such as an LDL-C receptor deficiency, which is commonly observed in certain types of familial hypercholesterolemia [11]; development of antidrug antibodies; genetic factors; and incorrect injection techniques. Moreover, the genetic polymorphism of the
The addition of a standard dose of statin in both reported cases allowed the resistance to PCSK9 inhibitors to be overcome with a dramatic end result. This result can be explained by statin effects, including reduction of hepatic intracellular cholesterol, activation of LDL-C receptors, and increases in circulating PCSK9. These effects enhance the use of PCSK9 inhibitors and potentiate the expected result. A 7.4% and 14% increase in the PCSK9 level after 6 weeks of treatment with 10 mg atorvastatin was reported by Mayne et al. [14] and Costet et al. [15], respectively, while Guo et al. [16] reported a 35% increase in PCSK9 level after 8 weeks of treatment with 20 mg atorvastatin [17]. This hypothesis is supported by the results of both of our cases, with LDL-C reduction being greater (76% reduction) in case 1 (PCSK9 inhibitors combined with 20 mg atorvastatin) than in case 2 (62% reduction, with PCSK9 inhibitors combined with 10 mg atorvastatin). Finally, these findings justify the systematic use of combined therapy (statin and PCSK9 inhibitors) for a better outcome in patients with LDL receptor abnormalities. Robinson et al. [5] showed that PCSK9 inhibitors are significantly more potent in patients on background statin therapy. Here, we arrived at the same conclusion from the other direction, by starting with a monotherapy and then adding alirocumab and statin. Prospective trials are needed to investigate the efficacy of monotherapy with the monoclonal antibodies PCSK9 inhibitors in the context of LDL-C receptor abnormalities and to determine if a genetic explanation underlies differences in interindividual responses.
Conclusions
Clinical trials have shown that PCSK9 inhibitors are effective as a monotherapy and in combined therapy. We presented 2 nonresponder cases in which the addition of a statin to treatment with PCSK9 inhibitors was essential to achieve the target reduction in LDL-C from the baseline level. Nonetheless, as PCSK9 inhibitors become more commonly prescribed, we expect that more cases of nonresponse will be identified. In patients with familial hypercholesterolemia who are deemed to be statin intolerant and have severely increased baseline LDL-C levels, monotherapy with PCSK9 inhibitors must be carefully considered. LDL-C apheresis may remain the optimal therapeutic approach for these patients.
References:
1.. Sabatine MS, Giugliano R, Keech AC, FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease: N Engl J Med, 2017; 376; 1713-22
2.. Saeed A, Virani SS, Jones PH, Case reports of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition nonresponse: J Clin Lipidol, 2018; 12; 1141-45
3.. Qamar A, Giugliano RP, Keech AC, Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab. An analysis of FOURIER trial data: JAMA Cardiol, 2019; 4(1); 59-63
4.. Sabatine MS, Giugliano RP, Keech AC, Evolocumab and clinical outcomes in patients with cardiovascular disease: N Engl J Med, 2017; 376; 1713-22
5.. Robinson JG, Farnier M, Kastelein JJP, Relationship between alirocumab, PCSK9, and LDL-C levels in four phase 3 ODYSSEY trials using 75 and 150 mg doses: J Clin Lipidol, 2019; 13(6); 979-88
6.. Kaufman TM, Warden BA, Minnier J, Application of PCSK9 inhibitors in practice. Part 2: The patient experience: Circ Res, 2019; 124; 32-37
7.. Norata GD, Tibolla G, Catapano AL, Targeting PCSK9 for hypercholesterolemia: Annu Rev Pharmacol Toxicol, 2014; 54; 273-93
8.. Mach F, Baigent C, Catapano AL, 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: Eur Heart J, 2020; 41(1); 111-88
9.. Zafrir B, Jubran A, Lipid-lowering therapy with PCSK-9 inhibitors in the real world setting: two-year experience of a regional lipid clinic: Cardiovasc Ther, 2018; 36(5); e12439
10.. Chamberlain AM, Gong Y, Shaw KM, PCSK9 inhibitor use in the real world: Data from the national patient-centered research network: J Am Heart Assoc, 2019; 8(9); e011246
11.. Thedrez A, Blom DJ, Ramin-Mangata S, Homozygous familial hyper-cholesterolemia patients with identical mutations variably express the LDLR (low-density lipoprotein receptor): implications for the efficacy of evolocumab: Arterioscler Thromb Vasc Biol, 2018; 38; 592598
12.. Saavedra YGL, Dufour R, Davignon J, Baass A, PCSK9 R46L, lower LDL, and cardiovascular disease risk in familial hypercholesterolemia: Arterioscler Thromb Vasc Biol, 2014; 34; 2700-5
13.. Raal FJ, Honarpour N, Blom DJ, Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolemia (TESLA Part B): A randomised, double blind, placebo-controlled trial: Lancet, 2014; 385; 341-50
14.. Mayne J, Dewpura T, Raymond A, Plasma PCSK9 levels are significantly modified by statins and fibrates in humans: Lipids Health Dis, 2008; 7; 22
15.. Costet P, Hoffmann MM, Cariou B, Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients: Atherosclerosis, 2010; 212; 246-51
16.. Guo YL, Liu J, Xu RX, Short-term impact of low-dose atorvastatin on serum proprotein convertase subtilisin/kexin type 9: Clin Drug Investig, 2013; 33; 877-83
17.. Nozue T, Lipid lowering therapy and circulating PCSK9 concentration: J Atheroscler Thromb, 2017; 24(9); 895-907
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250