21 July 2020: Articles 
High-Dose Prednisone for Treatment of Autoimmune Pancreatitis in a Patient with Coronavirus Disease 2019 (COVID-19) due to Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Unusual clinical course, Unusual setting of medical care
Hammad Liaquat1ADEF*, Brittney Shupp2BCEF, Sarina Kapoor1CDEF, Ayaz Matin1ADEFDOI: 10.12659/AJCR.926475
Am J Case Rep 2020; 21:e926475
Abstract
BACKGROUND: Autoimmune pancreatitis (AIP) is a rare, steroid-responsive disease of the pancreas. Concurrent treatment with immunosuppressants, including corticosteroids, increases the risk of developing a severe form of coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) advises against the use of corticosteroids in patients with SARS-CoV-2 due to their poor outcomes in patients with SARS-CoV and Middle East respiratory syndrome (MERS-CoV), unless these patients require steroid treatment for a coexisting disease.
CASE REPORT: A 53-year old patient was admitted with symptoms and diagnostic findings consistent with AIP. Thorough etiological workup revealed an elevated IgG4 level of 361 mg/dL and significant clinical response to corticosteroid treatment, leading to a diagnosis of AIP. After finishing steroid treatment at home, the patient was readmitted with another episode of AIP complicated by development of acute necrotic collection and COVID-19 while taking a second course of high dose prednisone. The patient was continued on high dose prednisone, started on azathioprine and intravenous meropenem, and underwent CT guided percutaneous drainage. He also received supportive care for COVID-19. After significant clinical improvement, the patient was discharged to quarantine at home, which he completed uneventfully.
CONCLUSIONS: Despite the use of corticosteroids due to AIP, this high risk patient recovered from COVID-19 without complications. These findings support the use of corticosteroids when necessary for treatment of coexisting conditions in COVID-19 patients.
Keywords: Autoimmune Diseases, COVID-19, Glucocorticoids, autoimmune pancreatitis, Betacoronavirus, COVID-19, Coronavirus Infections, Dose-Response Relationship, Drug, Pandemics, Pneumonia, Viral, Prednisone, SARS-CoV-2
Background
Autoimmune Pancreatitis (AIP) is a rare but distinct form of pancreatic disease that is responsive to first-line corticosteroids, as well as to immunomodulators [1]. Autoimmune conditions, along with advanced age, male gender, and preexisting comorbidities, are associated with increased risks of mortality and complications in patients with coronavirus disease 2019 (COVID-19) caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2–4]. Currently, the World Health Organization (WHO) advises against the administration of corticosteroids to patients with SARS-CoV-2 infection due to the poor outcomes observed with corticosteroids in patients with SARS-CoV and Middle East respiratory syndrome (MERS-CoV), unless corticosteroids are necessary for a coexisting disease [5,6]. This report describes a patient with symptomatic, complicated AIP who was receiving treatment with high dose corticosteroids and was diagnosed with COVID-19. This study illustrates the management of a patient with complex SARS-CoV-2 infection and AIP and the outcomes of corticosteroid treatment in this setting.
Case Report
A 53-year-old man with no prior medical history was admitted for evaluation of epigastric abdominal pain for 1 day which was radiating to the back, along with nausea. His serum lipase concentration was 2798 U/L and CT of the abdomen showed peripancreatic inflammation, consistent with a diagnosis of AIP. Etiological work up revealed no history of alcohol intake or cigarette smoking, no intake of any medications known to cause pancreatitis or any family history of pancreatitis or pancreatic cancer. He had normal calcium levels, borderline high triglyceride levels, and elevated IgG4 level of 361 mg/dL. Ultrasound and magnetic resonance cholangiopancreatography (MRCP) of his right upper quadrant were unremarkable. He was diagnosed with type 1 AIP due to elevated IgG4 levels, given supportive treatment and started on 40 mg/day prednisone. The patient recovered clinically soon after starting steroid treatment, further confirming the diagnosis of AIP, and he was discharged home. He was prescribed 40 mg/day prednisone for 30 days, followed by dose tapering of 5 mg/day per week.
Although the patient remained asymptomatic during treatment with corticosteroids, he was hospitalized with a second episode of AIP two weeks after completing corticosteroid treatment. He was provided supportive treatment and started on 40 mg/day prednisone. The patient reported improvement in abdominal pain and was able to tolerate a low fat diet. He was discharged from the hospital and prescribed 40 mg/day prednisone for 1 month, followed by dose tapering of 5 mg/day per week. He was also scheduled for an appointment at the gastroenterology clinic within one to two weeks after leaving the hospital.
One week after discharge from the hospitalization, the patient reported fever, a persistent dry cough, and malaise, along with unresolved abdominal pain and the recurrence of nausea. Cobas® SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) testing of a nasopharyngeal swab sample using the automated Cobas® 6800/8800 system showed that he was positive for SARS-CoV-2. He was admitted to the hospital, where he was found to be febrile, tachycardic, and tachypneic with normal blood pressure. Tenderness in the epigastric region was noted. His hematocrit was 35.8%, his white blood cell (WBC) count was 12.32×103/µL, his blood urea nitrogen (BUN) concentration was 9 mg/dl, his serum lipase concentration was 530 µ/L, and his liver function tests were normal. CT of the abdomen revealed severe pancreatitis along with a multiloculated heterogeneous accumulation of fluid and gas in the pancreatic and peripancreatic tissues, consistent with an infected acute necrotic collection (Figure 1A, 1B). Chest imaging showed bibasilar patchy ground glass opacities (Figure 2A) and the presence of nonspecific linear densities in the lower lung fields (Figure 2B), consistent with COVID-19 pneumonia.
The patient was continued on 40 mg/day prednisone and started on vitamin D3 and plaquenil for COVID-19. He was given single doses of ceftriaxone and flagyl to treat possible infected pancreatic necrosis. One day later, his antibiotic coverage was broadened and he was started on meropenem. The patient remained symptomatic and was unable to resume oral intake. He underwent CT guided percutaneous drainage of the pancreatic necrosis the next day. The following day, his pain improved and his diet was advanced. Following a 4-day course of meropenem and continued clinical improvement, the patient was transitioned to PO bactrim based on culture sensitivities of the pancreatic drainage. Due to the patient’s history of AIP relapse despite steroid therapy, 50 mg/day azathioprine was added to his discharge regimen, with a plan to wean off steroids by tapering his dose by 5 mg/day per week. After hospitalization for 7 days, the patient was discharged, prescribed bactrim for two additional days and instructed to quarantine at home. Despite continued high dose corticosteroid use in hospital, the patient did not require oxygen supplementation or experience any further complications from COVID-19.
Discussion
As the global COVID-19 pandemic unfolds, efforts are being made to identify individuals at highest risk of severe disease and to determine the proper treatment regimens for disease management. Multiple risk factors have been identified and associated with higher morbidity and mortality, especially in critically ill patients. Although the median age of patients with this disease has been shown to be approximately 56 years, individuals aged ≥64 years are at greatest risk for contracting this disease and experiencing complications from it [3,7]. Additionally, data from the Centers for Disease Control and Prevention have shown that individuals aged ≥45 years are at higher risk for hospitalization and ICU admission [2]. COVID-19 has also been shown to affect minorities more than white people, a difference likely due to disparities in healthcare, while also affecting males more than females [3]. However, one of the most significant risk factors associated with development of severe disease is having preexisting conditions, including cardiovascular disease, hypertension, diabetes, chronic lung disease, cancer, and diseases that require immunosuppressant therapy, such as corticosteroids [4,8]. AIP is a rare auto-immune disease that requires treatment with corticosteroids, placing these patients at greater risk of complications and mortality due to COVID-19.
AIP is a steroid responsive, pancreatic disease that is often evaluated and diagnosed after exclusion of other causes. There are two forms of AIP, characterized by the presence of extrapancreatic involvement and histological appearance [1,9]. Type 1 AIP, also called lymphoplasmocytic sclerosing pancreatitis (LPSP), is a systemic form of the disease that usually involves multiple organs and is associated with elevated IgG4 levels. Type 2 AIP, also called idiopathic duct centric pancreatitis (IDCP), is localized to the pancreas and is characterized by granulocyte epithelial lesions (GEL) due to infiltration of neutrophils into the pancreatic ductal epithelium. These two forms of AIP are similar, in that both are responsive to first-line corticosteroids, as well as to immunomodulators. Type 1 AIP usually presents in men aged >60 years as painless obstructive jaundice, with acute pancreatitis and presentation in younger persons considered rare. The diagnosis of AIP requires the presence of one or more components of the HISORt criteria (histology, characteristic findings on imaging, elevated IgG4 levels, involvement of other organs, and/or response of disease to corticosteroid usage) [1]. Our patient had elevated IgG4 levels and responded to corticosteroid therapy, factors consistent with a diagnosis of Type I AIP.
Our patient had severe AIP, complicated by the presence of an acute necrotic collection. In addition to inpatient treatment with high dose corticosteroids, he was managed by percutaneous drainage and treatment with antibiotics. This regimen is the most frequently used minimally invasive form of therapy, especially when compared with endoscopic drainage, because percutaneous drainage is successful in avoiding open necrosectomy, which is associated with higher morbidity and mortality rates [10]. If necessary, patients can be treated with additional minimally invasive procedures, such as endoscopic drainage to treat necrotic collections. These procedures are accompanied by the continued use of corticosteroids, which reduce pancreatic inflammation. Nevertheless, uncontrolled AIP and corticosteroids, which increaseing co-morbidity burden and immuno-suppression, respectively, place individuals at increased risk of severe COVID-19 infection [4,8]. Relatively little is known about the impact of gastrointestinal and liver diseases, specifically autoimmune conditions, on COVID-19 patients [11]. Because AIP is rare, it has not yet been implicitly discussed as a comorbid condition in COVID-19 patients, making management of this patient more complex and empiric.
Currently, the WHO advises that corticosteroids not be administered to patients with COVID-19, but does allow for their use in patients with preexisting conditions. Judicious use of steroids in the setting of COVID-19 was originally suggested in response to poor outcomes in previous viral outbreaks. such as SARS-CoV and MERS-CoV [5]. Although corticosteroids have been tested due to their potential ability to prevent inflammatory lung injury, their use was not advantageous and led to serious adverse reactions (Table 1). Patients with SARS-CoV and MERS-CoV who were treated with corticosteroids had higher plasma viral loads, suggesting delayed viral clearance due to corticosteroid impairment of the host immune system [5,12,13]. In addition, SARS-CoV patients treated with higher cumulative doses of corticosteroids were found to be more likely to develop complications, such as psychosis, diabetes, osteoporosis and avascular necrosis [14–16]. Systemic reviews of previous viral outbreaks and during the current SARS-CoV-2 pandemic have found that corticosteroid use was not advantageous in the past and requires further research to warrant their use for treatment of SARS-CoV-2 [17–19].
Few studies to date have investigated steroid use in COVID-19 illness, with those studies limited by small sample sizes and inconclusive or conflicting results (Table 2). In one study, a short course of low dose methylprednisolone improved outcomes in severe COVID-19 patients in the ICU by shortening supplemental oxygen use, reducing the need for mechanical ventilation and shortening ICU and hospitalization stay when compared with a control group [20]. Another study found that steroid treatment failed to show benefits in 15 critically ill patients with COVID-19 pneumonia [21], whereas other studies did not report definitive results [7,22,23]. Most of these studies are similar, in that they involve small sample sizes and administration of corticosteroids to critically ill patients, suggesting that they may be somewhat limited by the potential of confounding bias. Overall, these studies do not provide sufficient evidence to support routine corticosteroid use in COVID-19 [5,6]. However, the National Institute for Health and Care Excellence (NICE) has developed guidelines to specifically assist in the management of gastrointestinal and liver diseases during the COVID-19 pandemic [24]. These guidelines recommend that oral or rectal corticosteroids to treat compelling gastrointestinal or liver conditions not be discontinued when a patient is diagnosed with COVID-19. These guidelines are in accordance with WHO recommendations that allow for corticosteroid treatment of preexisting conditions in COVID-19 patients.
The outcomes of past studies, as well as the scarcity of data regarding steroid use in SARS-CoV-2 infected patients, have helped in the formulation of WHO recommendations on corticosteroid use in COVID-19 patients. The findings in our patient provide evidence supporting current WHO recommendations on the judicious use of corticosteroids for the treatment of preexisting conditions such as AIP in COVID-19 patients.
Conclusions
Corticosteroids are the first line of treatment for AIP. Little is known about the use of steroids to treat COVID-19 patients. Although corticosteroids resulted in poor outcomes in previous SARS-CoV and MERS-CoV outbreaks and are not recommended for use in patients with COVID-19, our patient did not experience additional COVID-19 complications despite having multiple risk factors (age >45 years, male gender, comorbidity due to uncontrolled AIP and steroid immunosuppression) associated with poor outcomes. The findings in our patient provide evidence supporting current recommendations that steroid therapy be administered for co-existing diseases in patients with COVID-19.
Figures
Tables
Table 1.. Studies demonstrating outcomes of corticosteroid use in patients with severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV).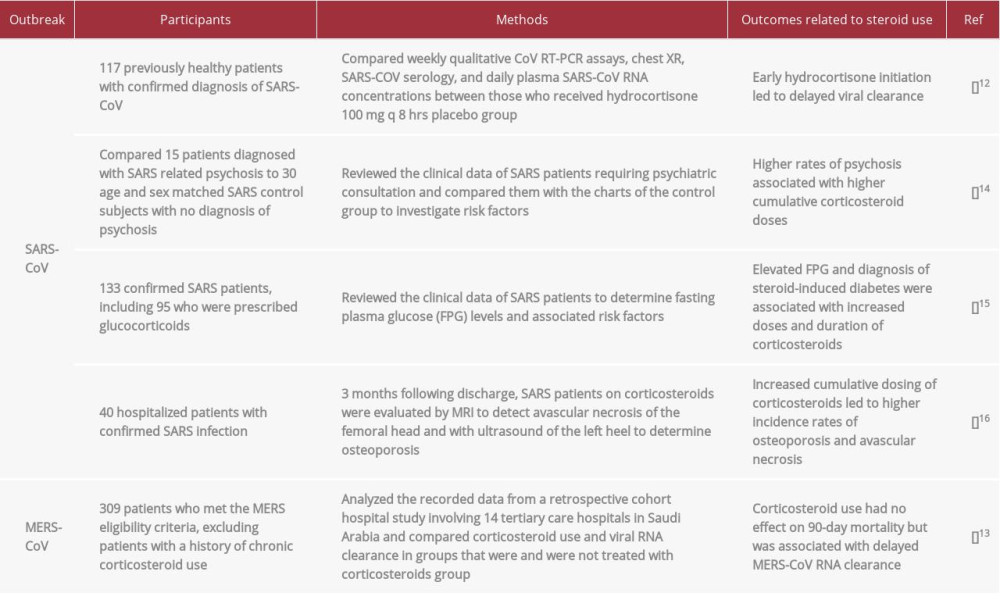 Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).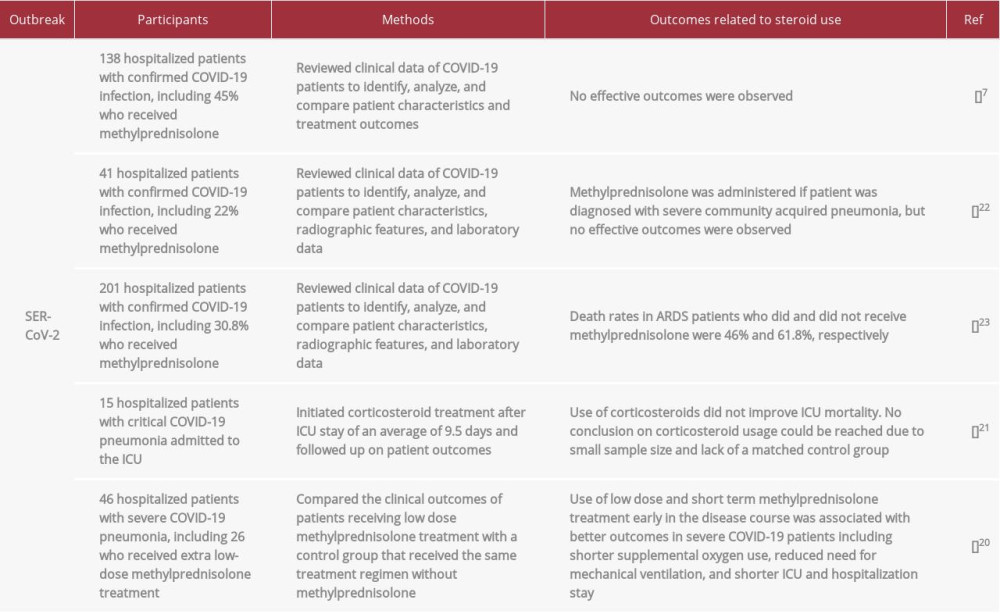
References:
1.. Nagpal SJS, Sharma A, Chari ST, Autoimmune pancreatitis: Am J Gastroenterol, 2018; 113; 1301
2.. , Severe outcomes among patients with coronavirus disease 2019 (COVID-19) – United States, February 12–March 16, 2020: Morb Mortal Wkly Rep, 2020; 69; 343-46
3.. Zhang J, Litvinova M, Wang W, Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: A descriptive and modelling study: Lancet Infect Dis, 2020; 20; 793-802
4.. Wang X, Fang X, Cai Z, Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: A systemic review and meta-analysis: Research (Wash D C), 2020; 2020; 2402961
5.. Russell CD, Millar JE, Baillie JK, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury: Lancet, 2020; 395; 473-75
6.. Lai CC, Shih TP, Ko WC, Severe acute respiratory syndrome corona-virus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges: Int J Antimicrob Agents, 2020; 55; 105924
7.. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323; 1061-69
8.. Favalli EG, Monti S, Ingegnoli F, Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: What can we learn from observational data?: Arthritis Rheumatol, 2020 [Online ahead of print]
9.. El Euch M, Hddad S, Mahfoudhi M, A case of type 1 autoimmune pancreatitis (AIP), a form of IgG4-related disease (IgG4-RD): Am J Case Rep, 2017; 18; 822-25
10.. Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology guideline: management of acute pancreatitis: Am J Gastroenterol, 2013; 108; 1400-15 , 1416
11.. Mao R, Liang J, Shen J, Chinese IBD Quality Care Evaluation Center Committee: Implications of COVID-19 for patients with pre-existing digestive diseases: Lancet Gastroenterol Hepatol, 2020; 5; 425-27
12.. Lee N, Chan KCA, Hui DS, Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients: J Clin Virol, 2004; 31; 304-9
13.. Arabi YM, Mandourah Y, Al-Hameed F, Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome: Am J Respir Crit Care Med, 2018; 197; 757-67
14.. Lee DTS, Wing YK, Leung HCM, Factors associated with psychosis among patients with severe acute respiratory syndrome: A case-control study: Clin Infect Dis, 2004; 39; 1247-49
15.. Xiao JZ, Ma L, Gao J, [Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy]: Zhonghua Nei Ke Za Zhi, 2004; 43; 179-82 [in Chinese]
16.. Li YM, Wang SX, Gao HS, [Factors of avascular necrosis of femoral head and osteoporosis in SARS patients’ convalescence]: Zhonghua Yi Xue Za Zhi, 2004; 84; 1348-53 [in Chinese]
17.. Veronese N, Demurtas J, Yang L, Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature: Front Med (Lausanne), 2020; 7; 170
18.. Ye Z, Wang Y, Colunga-Lozano LE, Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: A systematic review and meta-analysis: CMAJ, 2020 [Online ahead of print]
19.. Li H, Chen C, Hu F, Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: A systematic review and meta-analysis: Leukemia, 2020; 34; 1503-11
20.. Wang Y, Jiang W, He Q, A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia: Signal Transduct Target Ther, 2020; 5; 57
21.. Zhou W, Liu Y, Tian D, Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia: Signal Transduct Target Ther, 2020; 5; 18
22.. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395; 497-506
23.. Wu C, Chen X, Cai Y, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China: JAMA Intern Med, 2020 [Online ahead of print]
24.. : COVID-19 rapid guideline: gastrointestinal and liver conditions treated with drugs affecting the immune response, 2020, National Institute for Health and Care Excellence [updated 23 April 2020; cited 2020 12 June 2020]; https://www.nice.org.uk/guidance/ng172
Figures
Tables
 Table 1.. Studies demonstrating outcomes of corticosteroid use in patients with severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV).
Table 1.. Studies demonstrating outcomes of corticosteroid use in patients with severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV). Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Table 1.. Studies demonstrating outcomes of corticosteroid use in patients with severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV).
Table 1.. Studies demonstrating outcomes of corticosteroid use in patients with severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV). Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Table 2.. Studies demonstrating outcomes of corticosteroid treatment in patients with coronavirus disease 2019 (COVID-19) due to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








