02 October 2020: Articles 
Volumetric Flow Changes in Extracranial Arteries in a Symptomatic Patient with Significant Bilateral Carotid Artery Stenosis: A Case Study and Literature Review
Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Rare disease
Jerzy Leszczyński1ABCDEF, Piotr Kaszczewski1ABCDEF, Michał Elwertowski1ABCDE*, Kamil Stępkowski1BCD, Rafał Maciąg2BCD, Aleksandra Elwertowska1EF, Zbigniew Gałązka1ADEFDOI: 10.12659/AJCR.927202
Am J Case Rep 2020; 21:e927202
Abstract
BACKGROUND: Hemodynamically significant carotid artery stenoses are rarely diagnosed in people under 60 years of age, being mainly secondary to other concomitant diseases. Cerebral blood flow volume, which correlates with cerebrovascular reserve and susceptibility of ischemic symptoms occurrence, may aid in the diagnosis and monitoring of patients with carotid artery disease. In this report we present the case of a patient with significant bilateral carotid stenosis, focusing on the ultrasonographically measured changes in blood flow volume in extracranial arteries following surgeries.
CASE REPORT: A 41-year-old man with a positive history of transient ischemic attack (TIA) was referred to our department after being diagnosed with significant 80% to 85% right internal carotid artery (ICA) and 60% left ICA stenosis. After successful carotid endarterectomy, the flow volume in extracranial arteries significantly increased (from 755 mL/min to 1053 mL/min) due to an increase of flow volume in the right ICA. With the progression of left ICA stenosis, cerebral blood flow decreased, and the patient presented with a second TIA. Following the successful treatment of the left ICA stenosis (consisting of carotid endarterectomy and stent implantation because neointimal hyperplasia resulted in significant, recurrent 80% left ICA stenosis), an increase in flow volume was observed.
CONCLUSIONS: Assessment of the blood flow volume in extracranial arteries may be an effective tool in monitoring patients with carotid stenoses. Due to the lack of literature on this topic, further research on cerebral blood flow volume in surgical and non-surgical patients is needed to understand this phenomenon.
Keywords: Blood Volume, Blood Volume Determination, Carotid Artery Diseases, Ultrasonography, Doppler, Color, Carotid Artery, Internal, carotid stenosis, Endarterectomy, Carotid, Ischemic Attack, Transient
Background
Hemodynamically significant internal carotid artery (ICA) stenosis is estimated to be responsible for up to one-fifth of all ischemic strokes. The severity of stenosis, estimated on the basis of changes in peak systolic velocity (PSV) and end-diastolic velocity (EDV), is perceived as the most important risk factor for stroke among patients with carotid artery disease [1].
While the velocity thresholds and risk factors connected with plaque morphology and echogenicity are well characterized in the diagnosis of carotid stenosis, little attention is given to the flow volume in extracranial arteries [2,3]. Volumetric assessment of the flow in carotid and vertebral arteries may represent total cerebral blood flow, which strongly correlates with the cerebrovascular reserve and, therefore, with the risk of occurrence of ischemic symptoms [4,5].
It was recently confirmed that the flow volume in extracranial arteries may vary from low to very high values among patients with ICA stenosis [2].
A recent study including a large group of healthy individuals focused on normal reference values of cerebral blood flow [3]. Also, in a group of asymptomatic patients with hemodynamically significant carotid stenosis, it was shown that there are individuals in which blood flow volume in extracranial arteries is elevated and there are those in whom blood flow volume is lower than in healthy individuals. Elevated flow volumes are observed in other, often contralateral extracranial, arteries [6]. In one case study, the authors attempted to predict the outcome of an endarterectomy in a patient with critical ICA stenosis based on the angio-computed tomography (CT) and computational fluid dynamics technique [7].
Our previous research on the volumetric assessment of cerebral blood flow focused on assessing the potential risk of ischemic symptoms, including stroke, and identifying the patients with an elevated risk of such symptom occurrence [2,3,6]. While referencing the latest literature, we present a case report of a 41-year-old man who underwent 2 endarterectomies and stent implantations due to critical, symptomatic, bilateral, and eventually recurrent carotid stenosis. We focus on the flow volume changes in the operated arteries and the general flow redistribution in all extracranial arteries, which were observed during the treatment process.
Case Report
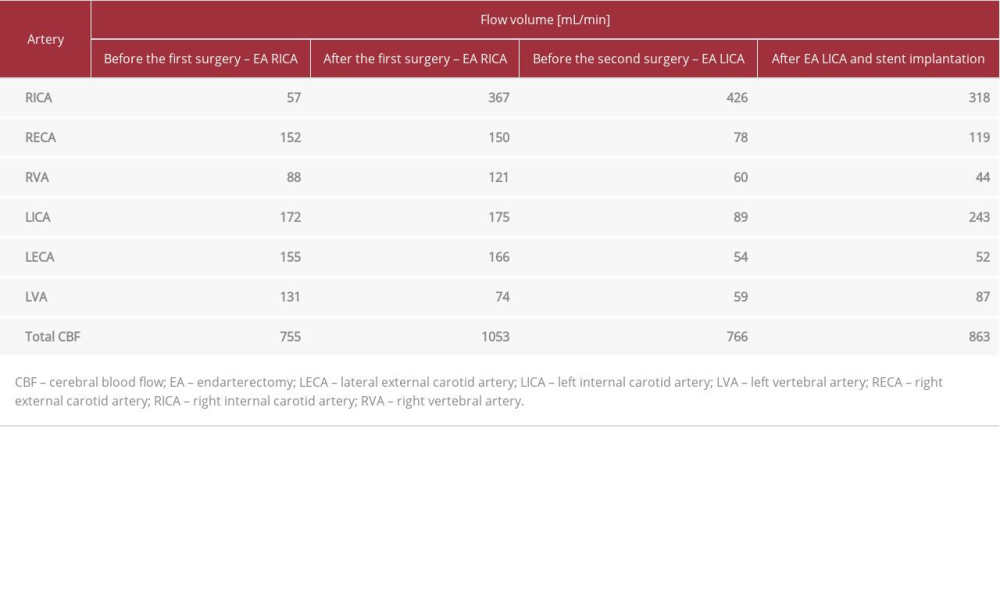
A 41-year-old man without concomitant disorders was referred to our department for surgical treatment with the diagnosis of symptomatic 80% to 85% right ICA stenosis. One month before hospitalization, the patient had a transient ischemic attack (TIA) with right-sided amaurosis fugax and left-sided loss of strength in the upper and lower extremities. The color Doppler duplex (CDD) examination, performed after the TIA episode, revealed 80% to 85% right ICA stenosis with a PSV of 3.8 m/s and EDV of 1.63 m/s, and significant flow reduction in the upper part of the artery, with PSV/EDV of 0.28/0.16 m/s. The 60% stenosis of the left ICA had flow velocities of PSV 2.96 m/s and EDV 1.11 m/s. The flow volumes were assessed in all extracranial arteries: the distal parts of both ICA, external carotid arteries (ECA) distally to the origin of superior thyroid arteries, and both vertebral arteries. The sum of the cerebral blood flow volume was 755 mL/min. The patient underwent an endarterectomy of the right ICA. The postoperative period was uneventful, and the patient was released from the hospital. The 4-month follow-up CDD examination after the endarterectomy showed a satisfactory treatment result. Cerebral blood flow volume had increased to 1053 mL/min because of the significant increase in right ICA flow volume (from 57 mL/min to 367 mL/min); the flow changes are shown in the Table 1 and Figure 1.
One year later, the patient was referred to our department because of the progression of left ICA stenosis to 80%, and we performed a left ICA endarterectomy. During the follow-up period, neointimal hyperplasia was observed, which after 1.5 years had caused recurrent 80% left ICA stenosis, with flow velocity values of PSV 2.62 m/s and EDV 1.09 m/s, distal reduction of the flow to PSV 0.42 m/s and EDV 0.22 m/s, and an ICA flow volume of 89 mL/min. Left-sided flow volume reduction caused a decrease of total cerebral blood flow to 766 mL/min. The patient had a TIA, which manifested itself as loss of consciousness. The patient was again qualified for surgical treatment. From the right femoral approach, carotid angioplasty was performed. A tapered Xact carotid stent 6/8×30 mm (Abbott, Abbott Park, IL, USA) was implanted with the use of an Emboshield Nav 6 distal neuroprotection system (Abbott). The postoperative period was uncomplicated. The follow-up CDD examination 4 months after the stent implantation confirmed the satisfactory result of the treatment and showed an increase in total cerebral blood flow to 863 mL/min, which was due to the significant increase in the flow volume in the left ICA. The increase in cerebral blood flow was not as prominent as after the first surgery because of the normalization of flow volume in the contralateral ICA. The changes in flow volume in the extracranial arteries are shown in Table 1 and Figure 2.
Following treatment, the patient remains under the direct supervision of our department and has remained asymptomatic to date.
Discussion
The prevalence of peripheral artery disease increases with age. Atherosclerosis is observed in about 20% of the population over 60 years old and in more than 50% over 85 years. Along with a continuously increasing life expectancy, the incidence of peripheral arterial disease is also expected to increase [8,9].
Carotid atherosclerotic lesions are observed in approximately 25% to 26% of the adult population, and thickening of the intima-media complex is seen in 9% to 12%. Stenotic plaques are observed in 1.5% to 2.7% of the general population, are more frequent in men, and have a strong correlation with age, starting from the fourth decade of life. In patients below the age of 39 years, they are rarely observed [10].
In young patients, advanced carotid artery disease is correlated with smoking, which is recognized as the strongest risk factor. Hyperlipidemia, family history, and associated coronary atherosclerosis are positively correlated with carotid atherosclerosis [11].
Several diseases, including B-thalassemia and Kawasaki disease, may be associated with the early development of carotid atherosclerosis and lead to hemodynamically significant, symptomatic ICA stenosis, which requires surgical treatment [12–14].
To prevent the occurrence of cerebrovascular ischemia and stroke due to surgical treatment, surgery should be performed only in select patients. According to the 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery, surgical treatment should be performed in symptomatic patients with more than 70% ICA stenosis [1]. The following randomized control trials also indicate that symptomatic patients with more than 70% ICA stenosis benefit most from surgical treatment: the Asymptomatic Carotid Atherosclerosis Study (ACAS), the Asymptomatic Carotid Surgery Trial (ACST-1), the North American Symptomatic Carotid Endarterectomy Trial (NASCET), and the European Carotid Surgery Trial (ECST). Symptomatic patients with more than 50% carotid stenosis may also benefit from surgery; therefore, it should also be considered for this group. The best results are obtained when the surgery is performed within 14 days of the occurrence of ischemic symptoms. Also, in asymptomatic patients with more than 70% ICA stenosis, surgical treatment should be performed [1,15].
Our patient presented with TIA twice, with symptoms including amaurosis fugax, loss of strength in the upper and lower extremities, and loss of consciousness. In such cases, surgical treatment is mandatory.
The surgical treatment is a safe and effective method in adults younger than 45 years of age. Mingoli et al. reported the results of carotid endarterectomy in a group of 1693 patients with more than 70% carotid stenosis, of which 49 patients were 35 to 45 years of age. The operative risk in this younger group of patients was low, and the 10-year disease-free interval was 75.7%. However, the authors identified a poor life expectancy in this group of patients, who usually die due to other complications of atherosclerosis [16].
Other authors have emphasized that young patients are far more prone to developing recurrent carotid stenosis; therefore, thorough follow-up with CDD should be performed [17,18].
Recurrent carotid stenosis is mainly associated with neointimal hyperplasia and the progression of the atherosclerotic process. Neointimal hyperplasia is responsible for early restenosis occurring less than 2 years after surgery, while the progression of the atherosclerotic process is responsible for cases occurring more than 2 years after surgery [19].
Neointimal hyperplasia is a pathological response of the arterial wall after the removal of a carotid plaque. It is associated with several processes, including thrombogenesis on an endarterectomized arterial wall, migration and growth of smooth muscle cells, accumulation of a proteoglycan-rich extracellular matrix, and increased activity of inflammatory cells. Intimal hyperplasia is more likely to occur after the dissection of stable carotid plaques, while removal of the soft, echolucent, and unstable plaques decreases the risk of early restenosis [20–22].
The treatment options for restenosis include open and endovascular methods. Both are associated with satisfactory long-term results. Stenting of recurrent carotid stenosis is safe and associated with high rates of technical success, allowing the attainment of 76% restenosis-free survival 4 years after surgery [23]. In our patient, neointimal hyperplasia occurred less than 2 years after the carotid endarterectomy of the left ICA, and was treated successfully with stent implantation, the patency of which needs to be checked regularly by CDD examination.
The issue of flow volume in extracranial arteries, which is not commonly assessed in patients with carotid stenoses, may have a vast spectrum of benefits in patients with carotid stenosis [2]. As presented in this case report, flow volume is connected with the severity of stenosis and may change after surgical treatment. Flow volume is also associated with the cerebrovascular reserve, which varies in symptomatic and asymptomatic patients [3].
The quantification of cerebral blood flow is challenging and is mainly achieved with radionuclide methods (positron emission tomography, single-photon emission CT, and Xenon-enhanced CT scanning), which are expensive and have limited availability [24,25].
Despite an ongoing debate on standards of cerebral blood flow, no studies have aimed to define standards of cerebral blood flow measured as the sum of the flow in the extracranial arteries. Only recently have such works appeared in the literature [3,26].
The literature has shown that sonographic quantification of cerebral blood flow is accurate, featuring high intradiane, interdiane, intraobserver and interobserver reproducibility, and comparability with radionuclides methods [3,4,24,27,28].
In physiological conditions, the ECA provide very little or no blood supply to the central nervous system. For this reason, most researchers do not include ECA in their assessment of cerebral blood flow. However, the ECA may become the vital collateral pathway when there is accompanying significant ICA stenosis. This collateralization might feature flow changes in the ECA: a decrease in flow resistance and an increase in EDV and flow volume. To assess the degree of compensation of the ECA, its flow volume should be known [3].
Sonographic volumetric flow assessment have several limitations. Concomitant disorders may influence the accuracy of measurements and include the following: uncontrolled hyper-tension, ischemic heart disease, heart insufficiency, positive history of heart infraction, positive history of stent implantation to coronary or any other arteries, cardiac arrhythmia, tachycardia, bradycardia, congenital vascular or heart failure, positive history of vascular interventions, and the presence of endocrine diseases such as thyroid goiter, hyperthyroidism, hypothyroidism, diabetes, adrenal diseases, positive history of thyroid surgery, smoking, and alcohol use. Therefore, we conduct all measurements using a previously described protocol [3] in patients without the aforementioned diseases. Specifically, we take 3 measurements of vessel diameter with 3 different imaging modalities, and 3 measurements of flow volume, with the final volume equaling the average of those measurements. The flow is measured in the distal part of the ICA, distal part of the ECA, distal origin of the superior thyroid artery, and in the V2 segment of the vertebral arteries, thereby minimizing the influence of turbulent flow and branching vessels (in the case of the ECA) on an overestimation of the results [2,3,6].
It is worth emphasizing that the ICA, ECA, and vertebral arteries are not the only branches supplying the central nervous system, but they are the largest caliber supplying vessels. Other small-caliber vessels include the ascending cervical arteries and arteriae cervicalis profundae. This may raise the question: why not include these arteries and other small-caliber arteries in the measurements? First, they are extremely difficult to visualize using standard sonographic techniques. Second, to obtain accurate volumetric flow data, the diameter of the vessel must be determined with maximum accuracy, which is not possible with deeply located small-caliber arteries and, therefore, may lead to substantial over- or underestimation of the flow volume [3,26]. Third, these vessels provide very little blood supply to central nervous system structures; therefore, even if included in the calculation, they might not produce statistically significant differences. Finally, it is also worth stressing that the abovementioned arteries are not included in CT or magnetic resonance imaging perfusion protocols.
The volumetric assessment of blood flow in the ECA with Doppler ultrasonography may be a source of additional information of the status of the patient with significant carotid stenosis.
Assessment of the blood flow in extracranial arteries may be an important tool for monitoring patients with not only carotid artery disease, but also with other neurological conditions.
Conclusions
Our study shows that surgical treatment of significant ICA stenosis influences blood flow volumetric changes, which may be strictly connected with reducing the risk of cerebral ischemia. The volumetric assessment of the blood flow in extracranial arteries may be a useful and effective tool in monitoring patients with carotid artery disease. However, to fully understand its role, further research on extracranial flow volumes in patients with different degrees of carotid stenosis is needed.
Figures
References:
1.. Aboyans V, Ricco JB, Bartelink ML, Editor’s Choice – 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Eur J Vasc Endovasc Surg, 2018; 55; 305-68
2.. Elwertowski M, Leszczyński J, Kaszczewski P, The importance of blood flow volume in the brain-supplying arteries for the clinical management – the impact of collateral circulation: J Ultrason, 2018; 18(73); 112-19
3.. Kaszczewski P, Elwertowski M, Leszczynski J, Volumetric carotid flow characteristics in Doppler ultrasonography in healthy population over 65 years old: J Clin Med, 2020; 9(5); E1375
4.. Scheel P, Ruge C, Petruch UR, Schoning M, Color duplex measurement of cerebral blood flow volume in healthy adults: Stroke, 2000; 31; 147-50
5.. Gupta A, Chazen JL, Hartman M, Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis [published correction appears in Stroke, 2013; 44(10): e137]: Stroke, 2012; 43(11); 2884-91
6.. Kaszczewski P, Elwertowski M, Leszczyński J, CAR 5. Carotid flow volume measurement in Doppler ultrasound as a new look at the diagnosis of internal carotid artery stenosis: J Vasc Surg, 2019; 70; e162
7.. Polanczyk A, Podgorski M, Wozniak T, Computational fluid dynamics as an engineering tool for the reconstruction of hemodynamics after carotid artery stenosis operation: A case study: Medicina (Kaunas, Lithuania), 2018; 54(3); 42
8.. Sigvant B, Wiberg-Hedman K, Bergqvist D, A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences: J Vasc Surg, 2007; 45; 1185-91
9.. Sigvant B, Lundin F, Wahlberg E, The risk of disease progression in peripheral arterial disease is higher than expected: A meta-analysis of mortality and disease progression in peripheral arterial disease: Eur J Vasc Endovasc Surg, 2016; 51; 395403
10.. Prati P, Vanuzzo D, Casaroli M, Prevalence and determinants of carotid atherosclerosis in a general population: Stroke, 1992; 23(12); 1705-11
11.. Rodman KD, Furlan AJ, Severe extracranial carotid atherosclerosis in young adults: J Stroke Cerebrovasc Dis, 1992; 2(3); 173-77
12.. Sherief LM, Dawood O, Ali A, Premature atherosclerosis in children with beta-thalassemia major: new diagnostic marker: BMC Pediatr, 2017; 17; 69
13.. Yokoya S, Tamura A, Hino A, Unusual clinical sequelae of Kawasaki disease-symptomatic extracranial internal carotid stenosis in young adult: World Neurosurg, 2018; 117; 162-64
14.. Kotsis T, Pappas E, Sarmas G, Carotid endarterectomy in a young symptomatic patient with B-thalassemia major: Ann Vasc Surg, 2015; 29(4); 838.e1-5
15.. Rothwell PM, Gutnikov SA, Warlow CP, Reanalysis of the final results of the European Carotid Surgery Trial: Stroke, 2003; 34(2); 514-2
16.. Mingoli A, Sapienza P, Feldhaus RJ, Carotid endarterectomy in young adults: Is it a worthwhile procedure?: J Vasc Surg, 1997; 25(3); 464-70
17.. Valentine RJ, Myers SI, Hagino RT, Clagett GP, Late outcome of patients with premature carotid atherosclerosis after carotid endarterectomy: Stroke, 1996; 27(9); 1502-6
18.. Levy PJ, Olin JW, Piedmonte MR, Carotid endarterectomy in adults 50 years of age and younger: A retrospective comparative study: J Vasc Surg, 1997; 25(2); 326-31
19.. Lal BK, Beach KW, Roubin GS, Restenosis after carotid artery stenting and endarterectomy: A secondary analysis of CREST, a randomised controlled trial: Lancet Neurol, 2012; 11(9); 755-63
20.. De Borst GJ, Moll F, Biology and treatment of recurrent carotid stenosis: J Cardiovasc Surg (Torino), 2012; 53; 27-34
21.. Hellings WE, Moll FL, De Vries JP, Atherosclerotic plaque composition and occurrence of restenosis after carotid endarterectomy [published correction appears in JAMA, 2009; 301(22): 2329]: JAMA, 2008; 299(5); 547-54
22.. Hellings WE, Moll FL, de Vries JP, Histological characterization of restenotic carotid plaques in relation to recurrence interval and clinical presentation: A cohort study: Stroke, 2008; 39(3); 1029-32
23.. de Borst GJ, Ackerstaff RG, de Vries JP, Carotid angioplasty and stenting for postendarterectomy stenosis: Long-term follow-up: J Vasc Surg, 2007; 45(1); 118-23
24.. Kalayci T, Sonmezgoz F, Apaydin M, Effects of carotid artery stenosis and plaque localization on the incidence of cerebral infarct and diameter of vertebral artery: A duplex ultrasonography and MRI evaluation: Int J Clin Exp Med, 2016; 9(11); 22393-97
25.. King A, Serena J, Bornstein NM, Markus HS, Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study: Stroke, 2011; 42; 1550-55
26.. Oktar SO, Yücel C, Karaosmanoglu D, Blood-flow volume quantification in internal carotid and vertebral arteries: Comparison of 3 different ultrasound techniques with phase-contrast MR imaging: Am J Neuroradiol, 2006; 27(2); 363-69
27.. Schöning M, Walter J, Scheel P, Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults: Stroke, 1994; 25; 17-22
28.. Schöning M, Scheel P, Color duplex measurement of cerebral blood flow volume: Intra- and interobserver reproducibility and habituation to serial measurements in normal subjects: J Cereb Blood Flow Metab, 1996; 16; 523-31
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250