28 October 2020: Articles 
Severe COVID-19 Pneumonia in a 30-Year-Old Woman in the 36 Week of Pregnancy Treated with Postpartum Extracorporeal Membrane Oxygenation
Unusual clinical course, Unusual or unexpected effect of treatment, Educational Purpose (only if useful for a systematic review or synthesis)
Watatu Takayama1ABCDEF*, Akira Endo1ABCE, Junichiro Yoshii1BCD, Hirokuni Arai2DEF, Keiji Oi2CDE, Eiki Nagaoka2CDE, Satoshi Toyama3CDE, Hiroto Yamamoto3CDE, Tokujiro Uchida3DEF, Yasuhiro Otomo1CDEFDOI: 10.12659/AJCR.927521
Am J Case Rep 2020; 21:e927521
Abstract
BACKGROUND: There are few reports of coronavirus disease 2019 (COVID-19) in pregnant women. Although coagulation dysfunction was reported to affect the severity of COVID-19, the association between pregnancy, which is usually accompanied by changes in coagulation function, and the worsening of COVID-19 is unknown. We present a case of a 30-year-old woman in the 36th week of pregnancy who was diagnosed with severe COVID-19 pneumonia and required postpartum extracorporeal membrane oxygenation (ECMO) therapy.
CASE REPORT: A 30-year-old, 36-weeks pregnant woman presented to our hospital and was diagnosed with severe COVID-19 pneumonia soon after she had undergone a cesarean section. Her respiratory failure could not be managed by conventional therapeutic approaches. Therefore, ECMO was administered on day 7. Controlling coagulation function to maintain ECMO therapy was challenging. Nafamostat mesylate and cryoprecipitate were administered to treat the hypercoagulative status and severe hypofibrinogenemia, respectively. Since coagulopathy and her respiratory state improved, the ECMO therapy was terminated on day 15.
CONCLUSIONS: We report a case of severe COVID-19 pneumonia in a pregnant woman urgently treated with ECMO in the postpartum period. Thus, this case highlights the importance of close monitoring and appropriate medical care for pregnant women with severe COVID-19 pneumonia.
Keywords: COVID-19, Extracorporeal Membrane Oxygenation, Puerperal Infection, Betacoronavirus, COVID-19, Cesarean Section, Coronavirus Infections, Pandemics, Pneumonia, Viral, Pregnancy, Pregnancy Complications, Infectious, SARS-CoV-2
Background
The novel coronavirus disease 2019 (COVID-19) is an emerging disease caused by the novel coronavirus called “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), with a rapid increase in cases since its first identification in Wuhan, China, in December 2019 [1,2]. In Japan, since the first diagnosis of COVID-19 on January 16th, 2020, the number of domestic patients has gradually increased and resulted in 75 218 cases and 1439 deaths [3].
Generally, pregnant women are at high risk of developing viral infections such as influenza-A, H1N1, severe acute respiratory syndrome coronavirus, and the Middle East respiratory syndrome coronavirus. Reports have indicated that SARS-CoV-2 is highly contagious and can be transmitted through droplets, which can trigger a cytokine storm and affect the functioning of multiple organs [4,5]. Nonetheless, it has been suggested that intrauterine transmission of SARS-CoV-2 is rare [6].
According to reports from the American College of Obstetricians and Gynecologists (ACOG) and the Centers for Disease Control (CDC), compared with non-pregnant women, pregnant women with COVID-19 in the US are at increased risk for severe symptoms and are more likely to be admitted to the intensive care unit (1.5% versus 0.9%) and to require mechanical ventilation (0.5% versus 0.3%). This suggests that pregnant women with COVID-19 should undergo careful follow-up to enable close monitoring and adequate management if symptoms become severe. Moreover, a large-population study in the United States reported that pregnancy with COVID-19 infection was associated with increased risk for ICU admission and administration of mechanical ventilation, but was not associated with mortality [6,7]. In contrast, another large study, in the United Kingdom, reported an increased risk of mortality (1.0%), stillbirth (1.2%), and preterm delivery (24.8%) in pregnant patients with COVID-19 [8,9].
It was reported that clotting function changes dramatically during pregnancy and postpartum, and the coagulation activity is approximately double that before pregnancy [10]. Moreover, several studies have reported the existence of a hypercoagulable state in severe COVID-19 that leads to multiple organ dysfunction and high mortality [11]. These combined pathophysiologies related to coagulation disorder might result in worsening outcomes of pregnant COVID-19 patients. Nonetheless, there are few reports on COVID-19 in pregnant women. Here, we report the case of a 30-year-old woman in the 36th week of pregnancy who needed immediate postpartum extracorporeal membrane oxygenation (ECMO) therapy for severe COVID-19 pneumonia.
Case Report
A 30-year-old woman at 36 weeks of gestation presented with fever and cough to a nearby general hospital where she was scheduled for delivery. Her symptoms started with 1 day of fatigue, followed by intermittent fever (38.0°C) and cough for 3 days. A nasopharyngeal swab test was positive for SARSCoV-2 by real-time reverse transcriptase polymerase chain reaction (RT-PCR) running on the cobas® 6800/8800 system and negative for other common respiratory viruses. She had no underlying diseases and all previous scheduled pregnancy screening test results were within normal limits. Her condition during diagnosis was relatively stable. She was diagnosed with mild pneumonia caused by SARS-CoV-2 and was subsequently admitted to the isolation room in a respiratory ward (Figure 1A). She had a pulse of 104 beats/min, a blood pressure of 114/80 mmHg, a respiratory rate of 24 breaths/ min, and oxygen saturation of 95% on room air. On day 8 after the onset of symptoms, her respiratory condition worsened, and she developed mild acute respiratory distress syndrome (ARDS) [12] (Figure 1B). Given the potential risks of impending respiratory decompensation and subsequent severe fetal hypoxia, an emergency cesarean section under combined spinal and epidural anesthesia was performed. A preterm female neonate weighing 2.56 kg was delivered uneventfully with an Apgar score of 8 (on day 8 after the onset of symptoms). The neonate was placed in isolation and observed at the Neonatal Intensive Care Unit. The result of RT-PCR testing for SARS-CoV-2 in the neonate was negative, and no other complications were observed. Several hours after the cesarean section, the mother was transferred to the general Intensive Care Unit (ICU) and intubated. The next day, she was transferred to our Emergency and Critical Care Center with a diagnosis of severe COVID-19 pneumonia (hospital day 1, day 9 after onset of symptoms).
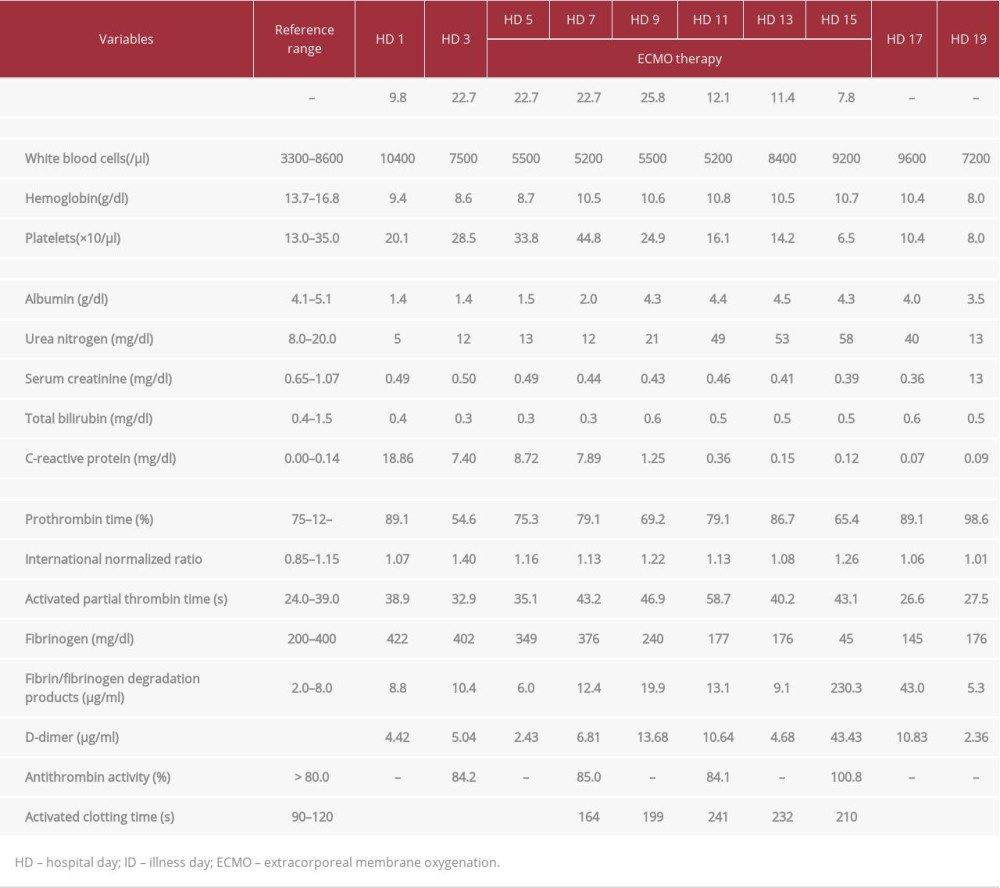
The initial physical examination revealed alert consciousness with a Glasgow Coma Scale of E4VTM6, body temperature of 37.7°C, blood pressure of 102/70 mmHg, pulse rate of 92 beats/min, respiratory rate of 28 breaths/min, and oxygen saturation of 89% under the mechanical ventilation setting including positive end-expiratory pressure (PEEP) of 12 and fractional inspired oxygen concentration (FIO2) of 50%. Her initial arterial blood gas analysis showed: pH 7.359, PaCO2 of 41.7 mmHg, PaO2 of 59.8 mmHg, HCO3– of 22.9 mmol/l, and lac-tate of 1.9 mmol/l, suggesting profound hypoxemia and moderate ARDS (Figure 1C). Other coagulation laboratory findings included fibrinogen (FIB), D-dimer, and fibrin-fibrinogen degradation products (FDP) of 422 mg/dl, 4.42 μg/ml, and 8.8 μg/ml, respectively. Levels of activated partial thrombin time (APTT) and prothrombin time international normalized ratio (INR) were 38.9 s and 1.07, respectively. After arrival at our hospital, favipiravir [13], which was approved by the Ethics Committee as a drug for treatment of COVID-19, was administered at a dose of 200 mg every 24 h for 2 weeks. Intravenous pulse methylprednisolone (500 mg/day) for ARDS was administered for 3 days. Anticoagulation with continuous unfractionated heparin infusion (UFH) was initiated for thrombosis prevention. In addition, tocilizumab [14], which is a suggested treatment option for COVID-19, was administered at a dose of 480 mg every 24 h on hospital days 4 and 5.
Her oxygenation gradually worsened after 6 days (hospital day 7); arterial blood gas showed pH of 7.404, PaO2 of 74.7 mmHg, PaCO2 of 47.2 mmHg under the mechanical ventilator setting, tidal volume of 333 ml (5.8 ml/kg predicted body weight), respiratory rate of 24 breaths/min, PEEP of 16, and FIO2 of 80% (severe ARDS and Murray score 3.5). In this setting, the plateau pressure increased up to 35 cmH2O. Transthoracic echocardiography excluded cardiogenic pulmonary edema resulting from a massive pulmonary embolism. Since her respiratory failure could not be managed by mechanical ventilation, medication, or prone positioning therapy, veno-venous ECMO was initiated on hospital day 7, which was the 15th day after the onset of symptoms, by following the Extracorporeal Life Support Organization guidelines [15]. ECMO was established via the right internal jugular vein for access (17 Fr cannula, inflow) and via the right femoral vein for return (24 Fr cannula, out-flow), with an initial blood flow of 4.5 L/min and sweep gas of 5.0 L (Capiox EBS SP-200 ”NEO” Terumo Corporation, Japan).
The mechanical ventilator was set at lung rest setting with driving pressure of 10, PEEP of 8, and FIO2 of 40%, and continuous diuretic administration, early nutrition, and rehabilitation were administered (Figure 1D). Additionally, high-dose intravenous immunoglobulin therapy was included from hospital day 7 to day 11. During ECMO therapy, target activated clotting time (ACT) and APTT were approximately 180 s and twice the reference value, respectively; however, although the level of anti-thrombin was within normal limits (<80%), her hypercoagulative status was not well-controlled by UFH at the dose of more than 15 U/kg/h. A duplex ultrasound confirmed the absence of deep vein thrombosis. To control coagulation function [16], simultaneously expecting a favorable effect on COVID-19 [17], nafamostat mesylate was administered from hospital day 7 onwards. From hospital day 10 onwards, chest radiography showed gradual improvement and lung compliance (Figure 1E).
On hospital day 11, severe hypofibrinogenemia (<50 mg/dl) was observed, and cryoprecipitate was administered for 2 days. As her respiratory condition had improved, ECMO therapy was terminated on hospital day 15, and the duration of ECMO support was 9 days. All procedures were performed safely without complications related to ECMO therapy, including circuit/oxygenator thrombosis, and no cannulation site hemorrhage occurred. She was extubated on hospital day 16 and subsequently could consume food orally without worsening of oxygenation, with an oxygen saturation of 98% under nasal cannula at 2L/min. Hypofibrinogenemia gradually improved with her respiratory condition and normalized on hospital day 19 (Figure 1F, Table 1). On hospital day 20, she was transferred to the previous hospital, where her baby was admitted. The patient and her infant had an uneventful postpartum and neonatal course. Her clinical course and treatments provided in our hospital are summarized in the progress chart (Figure 2).
Discussion
We report a case of severe COVID-19 pneumonia in a pregnant woman who was successfully treated with intensive care including early anticoagulation therapy, mechanical ventilation, and ECMO. Several studies have reported that pregnant women with viral respiratory illnesses are at higher risk than non-pregnant women of developing various complications, including critical ARDS and perinatal adverse outcomes, possibly due to changes in the immune system [1,2]. Previous studies have reported that fibrinogen and D-dimer elevation are associated with the severity of COVID-19 [18]. Moreover, it was reported that the fibrin level was elevated in the lungs of the COVID-19 patients, and excessive fibrin accumulation in the alveoli led to acute inflammation and pulmonary fibrosis [19]. It was reported that the coagulation system during pregnancy is complex, and standard coagulation tests such as INR and APTT have reported normal or slightly hypercoagulable states; however, some clotting factors (such as fibrinogen, factor VIII, IX, X, XII, and von Willebrand factor) are reported to be more than 2-fold higher during pregnancy than before pregnancy [10,20]. A large study reported an increased risk of mortality, stillbirth, and preterm delivery in pregnant patients with COVID-19 [8,9]. Therefore, it was speculated that pregnancy can affect the severity of COVID-19 pneumonia due to abnormalities in clotting function.
We encountered several challenging issues, especially in controlling the coagulation function to maintain ECMO therapy, although the relationship between coagulopathy and pregnancy was unclear. During ECMO therapy, nafamostat mesylate, which was reported to have increased in usage as a substitute for heparin because of its very short half-life [16] and thus potentially applicable for the treatment of COVID-19 [17], was used in addition to high-dose heparin to maintain ACT and APTT within the therapeutic range. However, the present case showed severe hypofibrinogenemia, possibly caused by administration of coagulation factor [21], tissue plasminogen activator-induced hyperfibrinolysis [17], or both, which can cause severe hemorrhage. Considering the risk of excessive fluid overload and lung edema, we used cryoprecipitate instead of fresh frozen plasma to prevent hemorrhagic complications related to COVID-19 [13,14].
Additionally, in this case, the timing and the anesthesia approach for cesarean section were considered to be debatable. The possibility of vertical transmission during vaginal delivery remains unknown, and COVID-19 itself is not an indication for immediate cesarean section. However, emergency cesarean section should be performed if there is intrauterine fetal distress caused by hypoxia or other reasons [22], as was observed in this case. To this end, multidisciplinary evaluation by an obstetrician, pulmonary physician, and an intensivist, would be required. Regarding the anesthetic approach, both neuraxial and general anesthesia have been reported to be safe in pregnant patients [23]. However, a study recommended that cesarean section under regional anesthesia should be avoided in COVID-19 patients with respiratory symptoms or hypoxemia [22], since regional anesthesia could cause hypo-tension, possibly due to activation of angiotensin-converting enzyme II receptor [24]. As our patient had already developed ARDS prepartum, cesarean section under general anesthesia with intubation and mechanical ventilation might have been a therapeutic option, with possible benefit from changes in cardiopulmonary physiology.
The relationship between pregnancy and the worsening of COVID-19 cannot be discussed without epidemiologic comparison; however, in addition to a previous report of pregnant COVID-19 cases [25], the present case has some remarkable implications regarding the progressive nature of COVID-19 in pregnant women, the interaction between pregnancy-related hypercoagulability and that induced by COVID-19, and, possibly, an appropriate anesthetic approach for cesarean section.
Conclusions
We report the case of a 30-year-old woman in the 36th week of pregnancy who developed severe COVID-19 pneumonia and required ECMO therapy postpartum. This case suggests that SARS-CoV-2 infection during pregnancy might indeed entail high risk of ARDS and coagulation disorder, and thus would require respiratory support. Thus, our present report supports the view that pregnant women with COVID-19 pneumonia require close follow-up to provide prompt treatment if necessary. Further reports are needed to describe the clinical characteristics and treatment approaches of COVID-19 in pregnant women.
Figures
References:
1.. Chen N, Zhou M, Dong X, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study: Lancet, 2020; 395(10223); 507-13
2.. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323(11); 1061-69
3.. , Coronavirus (COVID-19) News, 2020 https://news.google.com/covid19/map?hl=enUS&mid=%2Fm%2F03_3d&gl=US&ceid=US%3Aen
4.. Li X, Geng M, Peng Y, Meng L, Lu S, Molecular immune pathogenesis and diagnosis of COVID-19: J Pharm Anal, 2020; 10(2); 102-8
5.. Nkeih C, Sisti G, Schiattarella A, Elevated transaminases in a COVID 19-positive patient at term of gestation: A case report: Covid-19 and pregnancy: Acta Biomed, 2020; 91(3); e2020002
6.. , Statement on COVID-19 and Pregnancy June 24; 2020 https://www.acog.org/news/news-releases/2020/06/acogstatement-on-covid-19-and-pregnancy
7.. Elkafrawi D, Joseph J, Schiattarella A, Intrauterine transmission of COVID-19 in Pregnancy: Case report and review of literature: Acta Biomed, 2020; 91(3); e2020041
8.. Ellington S, Strid P, Tong VT, Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status– United States, January 22–June 7, 2020: MMWR Morb Mortal Wkly Rep, 2020; 69(25); 769-75
9.. Knight M, Bunch K, Vousden N, Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National Population Based Cohort Study: BMJ, 2020; 369; m2107
10.. Katz D, Beilin Y, Disorders of coagulation in pregnancy: Br J Anaesth, 2015; 115; 75-88
11.. Tang N, Li D, Wang X, Sun Z, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia: J Thromb Haemost, 2020; 18(4); 844-47
12.. Ranieri VM, Rubenfeld GD, Thompson BT, Acute respiratory distress syndrome: The Berlin Definition: JAMA, 2012; 307(23); 2526-33
13.. Yamamura H, Matsuura H, Nakagawa J, Effect of favipiravir and an anti-inflammatory strategy for COVID-19: Crit Care, 2020; 24; 413
14.. Biran N, Lp A, Ahn J, Tocilizumab among patients with COVID-19 in the Intensive Care Unit: A multicentre observational study: Lancet Rheumatol, 2020; 2(10); e603-12
15.. , Extracorporeal Life Support Organization guidelines, 2020 https://www.elso.org/Resources/Guidelines.aspx
16.. Han SJ, Kim HS, Kim KI, Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation: J Korean Med Sci, 2011; 26; 945-50
17.. Yamamoto M, Kiso M, Saka-Tagawa Y: bioRxiv, 2020; 2020; 054981
18.. Zou Y, Guo H, Zhang Y, Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China: Biosci Trends, 2020; 14(4); 285-89
19.. Gralinski LE, Bankhead A, Jeng S, Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury: mBio, 2013; 4(4); e00271-13
20.. Szecsi PB, Jørgensen M, Klajnbard A, Haemostatic reference intervals in pregnancy: Thromb Haemost, 2010; 103; 718-27
21.. McVeen RV, Lorch V, Carroll RC, Letter to the editor: Changes in fibrinolytic factors in newborns during extracorporeal membrane oxygenation (ECMO): Am J Hematol, 1991; 38; 254-55
22.. Chen R, Zhang Y, Huang L, Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: A case series of 17 patients: Can J Anesth, 2020; 67; 655-63
23.. Birnbach DJ, Bateman BT, Obstetric anesthesia: Leading the way in patient safety: Obstet Gynecol Clin N Am, 2019; 46; 329-37
24.. Zhou P, Yang XL, Wang XG, A pneumonia outbreak associated with a new coronavirus of probable bat origin: Nature, 2020; 579(7798); 270-73
25.. Fiore A, Piscitelli M, Adodo DK, Successful use of extracorporeal membrane oxygenation postpartum as rescue therapy in a woman with COVID-19: J Cardiothorac Vasc Anesth, 2020 [Online ahead of print]
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250