03 October 2020: Articles 
A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma
Unusual clinical course
Joanna L. Moore12EF*, Pavan V. Ganapathiraju3EF, Caroline P. Kurtz4E, Booth Wainscoat5EDOI: 10.12659/AJCR.927812
Am J Case Rep 2020; 21:e927812
Abstract
BACKGROUND: This is a case report of an immunocompromised patient with a history of non-Hodgkin lymphoma and persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who was seronegative and successfully treated with convalescent plasma.
CASE REPORT: A 63-year-old woman with a past medical history of non-Hodgkin lymphoma in remission while on maintenance therapy with the anti-CD20 monoclonal antibody, obinutuzumab, tested positive for SARS-CoV-2 via nasopharyngeal reverse transcription polymerase chain reaction (RT-PCR) testing over 12 weeks and persistently tested seronegative for immunoglobulin G (IgG) antibodies using SARS-CoV-2 IgG chemiluminescent microparticle immunoassay technology. During this time, the patient experienced waxing and waning of symptoms, which included fever, myalgia, and non-productive cough, but never acquired severe respiratory distress. She was admitted to our hospital on illness day 88, and her symptoms resolved after the administration of convalescent plasma.
CONCLUSIONS: As the understanding of the pathogenesis of SARS-CoV-2 continues to evolve, we can currently only speculate about the occurrence of chronic infection vs. reinfection. The protective role of antibodies and their longevity against SARS-CoV-2 remain unclear. Since humoral immunity has an integral role in SARS-CoV-2 infection, various phase 3 vaccine trials are underway. In the context of this pandemic, the present case demonstrates the challenges in our understanding of testing and treating immunocompromised patients.
Keywords: Antibodies, Coronavirus, Coronavirus Infections, COVID-19, Immunocompromised Host, Serologic Tests, Antibodies, Monoclonal, Humanized, Antineoplastic Agents, Immunological, COVID-19, COVID-19 Testing, Clinical Laboratory Techniques, Follow-Up Studies, Immunization, Passive, Lymphoma, Non-Hodgkin, Pandemics, Pneumonia, Viral, Real-Time Polymerase Chain Reaction, Severity of Illness Index
Background
In December 2019, hospitals in Wuhan, China, began reporting cases of severe pneumonia of unknown etiology [1]. Most of the initial identified patients were geographically linked to a local wet seafood market, where living and dead wild animals were sold. The virus rapidly spread to over 200 countries and territories [2]. Further sampling and genomic sequencing of respiratory tract specimens identified a novel coronavirus, severe acute respiratory syndrome coronavirus type 2 (SARSCoV-2), causing the 2019 pandemic known as coronavirus disease 2019 (COVID-19) [1]. Although mild upper respiratory tract infection characterizes most cases of COVID-19, severe outcomes have recently been identified in patients with secondary comorbidities [1]. Hypertension, cardiovascular diseases, and diabetes have been highlighted as potential risk factors for severe outcomes in COVID-19, as serum analysis of patients with these chronic diseases demonstrate higher levels of proinflammatory cytokines [1].
There is currently no known cure for COVID-19 disease; however, several COVID-19 vaccine trials are underway. Convalescent plasma is emerging as a potential therapy for COVID-19 because of its efficacy in treating previous viral infections such as Ebola, severe acute respiratory syndrome (SARS-CoV-1), and influenza A H1N1 [3]. To date, there is only anecdotal evidence to support the success of convalescent plasma use. However, there are 47 ongoing studies on convalescent plasma, including 22 randomized control trials, which will shed light on this potential therapy and help advance the development of a vaccine [4].
As our understanding of the pathogenesis of SARS-CoV-2 infection continues to evolve, and in the absence of a proven therapy or vaccine, diagnostic testing is a valuable tool to identify and isolate positive COVID-19 cases, especially in the immunocompromised population. Despite public health officials implementing extreme measures to isolate COVID-19 cases, most countries, including the United States, have been underprepared and, as a result, faced community transmission before testing was readily available to optimize early containment. The testing measures used in this case report included viral DNA detection and serological testing. The Sunrise Medical Laboratory obtained FDA emergency use authorization (EUA) as an established laboratory under the guidelines of the World Health Organization, with the cognizance of the possibility of false positives and false negatives of its nasopharyngeal reverse transcription polymerase chain reaction (RT-PCR) test (
This report describes the case of an immunocompromised woman with a history of non-Hodgkin lymphoma with persistent SARS-CoV-2 infection, revealed by nasopharyngeal RT-PCR, who was seronegative and successfully treated with convalescent plasma.
Case Report
This is the case of a 63-year-old woman with a past medical history of non-Hodgkin lymphoma in remission while on maintenance therapy with the anti-CD20 monoclonal antibody, obinutuzumab. She developed symptoms 37 days following her last dose of obinutuzumab after exposure to a close contact who tested positive for SARS-CoV-2. The patient experienced fever, myalgia, and non-productive cough and subsequently tested positive for SARS-CoV-2 infection a few days later via nasopharyngeal RT-PCR testing from Sunrise Medical Laboratories. After 19 additional days of persistent symptoms, the patient presented to the emergency department (ED). Because the patient was stable, she was discharged from the ED to continue self-monitoring while home quarantined. With unresolving symptoms and the onset of chest palpitations, the patient presented to the ED again, then 32 days into illness, and was found to have new onset of atrial fibrillation with a rapid ventricular response. A computed tomography (CT) angiogram of the chest was performed and revealed no pulmonary embolism; however, there were new bibasilar rounded ground-glass opacities compared to a routine CT for lymphoma surveillance performed approximately 1 month prior to the onset of her initial symptoms (Figure 1). The patient was discharged home after receiving metoprolol for heart rate regulation and apixaban for anticoagulation. During quarantine adherence at home, the patient continued to feel symptomatic, and outpatient nasopharyngeal RT-PCR SARS-CoV-2 nucleic acid amplification testing was persistently positive on days 34, 48, and 74 of illness and undetectable for SARS-CoV-2 IgG antibodies on illness days 41 and 75. The patient was admitted to our hospital in June 2020, on day 88 of illness, and was administered convalescent plasma as per the Mayo Clinic’s Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19 national clinical trial (NCT identifier: 04338360). One unit (approximately 200 mL) of compatible plasma was donated by COVID-19 survivors who had a confirmed laboratory diagnosis and had been symptom free for 14 days or more, with 1 aliquot removed to confirm SARSCoV-2 antibody presence by Ortho-Clinical Diagnostics VITROS Anti-SARS-CoV-2 IgG chemiluminescence immunoassay test [8].
On admission, the physical examination revealed that the patient had bilateral rhonchi and oxygen saturation of 100% on room air and was afebrile with a temperature of 37.1°C. Laboratory findings obtained on admission showed a normal leukocyte count with neutrophilic predominance and lymphopenia. Renal function testing was normal, and LDH and C-reactive protein levels were mildly elevated. The patient’s serum on illness day 88 was undetectable for IgG, and after the administration of plasma, repeat IgG testing was negative on day 89. Cryptococcal antigen, QuantiFERON, galactomannan, and Fungitell 1,3 beta-glucan assay tests were negative (Table 1). After convalescent plasma administration, the patient’s symptoms resolved, and she remained asymptomatic at a telemedicine follow-up visit 1 week later.
Discussion
SARS-CoV-2 is a Coronaviridae family non-retroviral RNA virus with large clinical diversity [9]. The family of coronaviruses (CoVs) has 4 subdivisions: alpha, beta, gamma, and delta [10]. SARS-CoV-2 is a member of the beta subdivision, along with the Middle East Respiratory Syndrome coronavirus and SARSCoV-1 [11]. The outer phospholipid bilayers of all CoVs contain a spike glycoprotein trimmer (S), a membrane protein (M), and an envelope protein (E), which surround the genomic RNA and a phosphorylated nucleocapsid protein (N) [12]. Unique to SARSCoV-2 is the longer and more molecularly divergent S proteins, which binds to human angiotensin-converting enzyme 2 (ACE2) in order for the virus to enter host cells [1]. In comparison to SARS-CoV-1, SARS-CoV-2 binds to ACE2 receptors with up to a 20-fold higher affinity, likely explaining the increased speed of human-to-human transmission [13]. Organs with increased expression of ACE2 receptors have a higher risk of SARS-COV-2 infection, such as the salivary glands, lung, heart, esophagus, kidney, bladder, ileum, testis, and brain, implicating many of the multi-organ complications that have been witnessed during this pandemic [14,15].
Upon entry into the cell via ACE2 receptors, the SARS-CoV-2 virus causes a severe inflammatory response by releasing higher levels of interleukin (IL)-2R, IL-6, IL-10, IL-17, and tumor necrosis factor-α [16,17]. It has been proposed that the mechanism of the inflammatory response to SARS-CoV-2 is biphasic, whereby, in the primary phase, active viral replication causes endothelial and epithelial cell apoptosis and vascular leakage, triggering a release of proinflammatory cytokines and chemokines [18]. In addition, pyroptosis, a highly inflammatory form of programmed cell death, occurs in macrophages and lymphocytes [18]. The S protein also downregulates ACE2 and induces ACE2 shedding during the primary phase, and loss of ACE2 function can cause acute lung injury and dysfunction of the renin-angiotensin system [19–21]. Renin-angiotensin system dysfunction then increases vascular permeability and inflammation. The secondary phase consists of adaptive immunity activation and the generation of neutralizing antibodies [18]. Neutralizing antibodies binding to protein S can alter inflammatory responses, leading to diffuse alveolar damage, although this mechanism remains unclear [22]. Immunocompromised patients may also have antibody-dependent enhancement during this phase. Antibody-dependent enhancement has been seen in dengue, influenza, and flavivirus and is a phenomena of virus-antibody immunocomplexes binding to cells with complement or Fc receptors promoting viral cell uptake [18,23]. Our understanding of the biological response to SARS-CoV-2 infection and the host’s immunological status is limited, as demonstrated by reports both refuting and supporting immunosuppression as a risk factor for more fatal COVID-19 outcomes [24].
Typically, non-retroviral RNA viruses have rapid viral replication and shedding presenting as an acute infection followed by a recovery phase, after which the individual has immunity against reinfection for varying periods of time [9]. Some non-retroviral RNA viruses, such as hepatitis C and some Coronaviridae viruses, have developed mechanisms to allow for persistent infections, although this has not yet been seen in SARS-CoV-2 [9]. Persistent viral infections resulting in chronic and recurrent pneumonias are rare. Recurrent pneumonia is defined as 2 or more distinct episodes of lower respiratory tract infections separated by either clearing visible on chest X-ray or a 1-month asymptomatic period, whereas chronic pneumonia lasts at least 6 weeks and is secondary to an infection [25]. The immune response to SARS-CoV-2 infection requires the activation of the innate and acquired immune systems in the biphasic response, as described above. The overactivation of T cells with a subsequent increase in TH17 and CD8+ T cells has been observed to be related to severe lung injury [18]. Since an immune response that is in overdrive may be the primary cause of organ damage, the anti-inflammatory effects of immuno-suppression may be protective against the cytokine storm related to COVID-19 outcomes [26].
The present case illustrates the dilemma of SARS-CoV-2 testing in immunocompromised patients. Although obinutuzumab has a half-life of only 28 days, this patient had symptoms persisting up to 126 days after immunotherapy administration [27]. Early in this pandemic, the oncological population was identified as an at-risk population [28]. Questions arose, for instance, regarding patients on immunosuppressive therapy and whether treatment should be delayed or used in conjunction with antibody testing prior to proceeding with chemotherapy or immunotherapy, such as in our patient with non-Hodgkin lymphoma [29]. It is unclear if this patient was re-infected after an initial period of improvement or, given her immunocom-promised status, if this was a persistent infection with variable immune responses to the administration of obinutuzumab.
Furthermore, most testing of SARS-CoV-2 viral RNA has been via PCR testing, and given that such tests are relatively new, it is important to note that the actual clinical sensitivity and specificity of these tests are unknown, further complicating the understanding of the true disease state of an individual with false positive and false negative results, especially in the immunocompromised population. Serological testing is still being developed and optimized and has limited availability. As laboratories continue to develop better immunoassays, further research is required to refine the current methods, and multiple tests need to be standardized on an international level to provide the adequate data reporting needed to appropriately contain the pandemic.
The patient’s lack of antibody response several days into her course of illness is also concerning, perhaps placing her at increased risk of repeat or persistent infection. Recent data suggest that SARS-CoV-2 IgG antibodies may have a more accelerated decay than SARS-CoV-2, and that the rate varies among those with mild
Conclusions
The present case demonstrates the difficulty in testing and treating immunocompromised patients for SARS-CoV-2 infection. Inconsistent observations show that the knowledge about the relationship between SARS-CoV-2 and host immune response status is limited. Further studies are required to elucidate the immune responses and inflammatory features of SARS-CoV-2 infection. According to the guidelines of the Centers for Disease Control, immunocompromised patients remain as high-risk to SARS-CoV-2 [31]. Close follow-up of these patients is required until more evidence and results from randomized control trials are available in this population. Until a validated viral culture is readily available and further studies truly define the protection and rate of decline of antiviral antibodies beyond 90 days, we can only postulate whether a chronic infectious state is possible in this disease [31,32].
References:
1.. Zhou P, Yang X, Wang X, A pneumonia outbreak associated with a new coronavirus of probable bat origin: Nature, 2020; 579; 270-73
2.. Gao Z, Xu Y, Sun C, A systematic review of asymptomatic infections with COVID-19: J Microbiol Immunol Infect, 2020 [Online ahead of print]
3.. Chen L, Xiong J, Bao L, Shi Y, Convalescent plasma as a potential therapy for COVID-19: Lancet Infect Dis, 2020; 20(4); 398-400
4.. Valk SJ, Piechotta V, Chai KL, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review: Cochrane Database Syst Rev, 2020; 5(5); CD013600
5.. , Use of laboratory methods for SARS diagnosis, 2020 https://www.who.int/csr/sars/labmethods/en/
6.. Yang HS, Racine-Brzostek SE, Lee WT, SARS-CoV-2 antibody characterization in emergency department, hospitalized and convalescent patients by two semi-quantitative immunoassays: Clin Chim Acta, 2020; 509; 117-25
7.. Long QX, Liu BZ, Deng HJ, Antibody responses to SARS-CoV-2 in patients with COVID-19: Nat Med, 2020; 26(6); 845-48
8.. Joyner M, Senefeld J, Klassen S, Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience: medRxiv, 2020; 2020; 20169359
9.. Alphalhão M, Ferreira JA, Filipe P, Persistent SARS-CoV-2 infection and the risk for cancer: Med Hypotheses, 2020 [Online ahead of print]
10.. Morty RE, Ziebuhr J, Call for papers: The pathophysiology of COVID-19 and SARS-CoV-2 infection: Am J Physiol Lung Cell Mol Physiol, 2020; 318(5); L1016-19
11.. , The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2: Nat Microbiol, 2020; 5(4); 536-44
12.. Li G, Fan Y, Lai Y, Coronavirus infections and immune responses: J Med Virol, 2020; 92(4); 424-32
13.. Wrapp D, Wang N, Corbett KS, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation: Science, 2020; 367(6483); 1260-63
14.. Zou X, Chen K, Zou J, Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection: Front Med, 2020; 14(2); 185-92
15.. Wang Z, Xu X, scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in Spermatogonia, Leydig and Sertoli Cells: Cells, 2020; 9(4); 920
16.. Zhang J, Wang X, Jia X, Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China: Clin Microbiol Infect, 2020; 26(6); 767-72
17.. Wu D, Yang XO, TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib: J Microbiol Immunol Infect, 2020; 53(3); 368-70
18.. Fu Y, Cheng Y, Wu Y, Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools: Virol Sin, 2020; 35(3); 266-71
19.. Glowacka I, Bertram S, Herzog P, Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63: J Virol, 2010; 84(2); 1198-205
20.. Haga S, Yamamoto N, Nakai-Murakami C, Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry: Proc Natl Acad Sci USA, 2008; 105(22); 7809-14
21.. Imai Y, Kuba K, Rao S, Angiotensin-converting enzyme 2 protects from severe acute lung failure: Nature, 2005; 436(7047); 112-16
22.. Liu L, Wei Q, Lin Q, Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection: JCI Insight, 2019; 4(4); e123158
23.. Taylor A, Foo SS, Bruzzone R, Fc receptors in antibody-dependent enhancement of viral infections: Immunol Rev, 2015; 268(1); 340-64
24.. Martins-Chaves RR, Gomes CC, Gomez RS, Immunocompromised patients and coronavirus disease 2019: A review and recommendations for dental health care: Braz Oral Res, 2020; 34; e048
25.. Geppert EF, Chronic and recurrent pneumonia: Semin Respir Infect, 1992; 7(4); 282-88
26.. Romanelli A, Mascolo S, Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: A therapeutical hypothesis: Am J Transplant, 2020; 20(7); 1947-48
27.. Sachdeva M, Dhingra S, Obinutuzumab: A FDA approved monoconal antibody in the treatment of untreated chronic lymphocytic leukemia: Int J Appl Basic Med Res, 2015; 5(10); 54-57
28.. Patel R, Park J, Shah A, COVID-19 and cancer patients: Cancer Med J, 2020; 3(1); 40-48
29.. Passaro A, Peters S, Mok TSK, Testing for COVID-19 in lung cancer patients: Ann Oncol, 2020; 31(7); 832-34
30.. Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Rapid decay of anti-SARSCoV-2 antibodies in persons with mild Covid-19: N Engl J Med, 2020; 383(11); 1085-87
31.. : Criteria for releasing COVID-19 patients from isolation: scientific brief, 2020, World health Organization
32.. Johnson KM, Belfer JJ, Peterson GR, Managing COVID-19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid-sparing immunosuppression: Pharmacotherapy, 2020; 40(6); 517-24
Tables
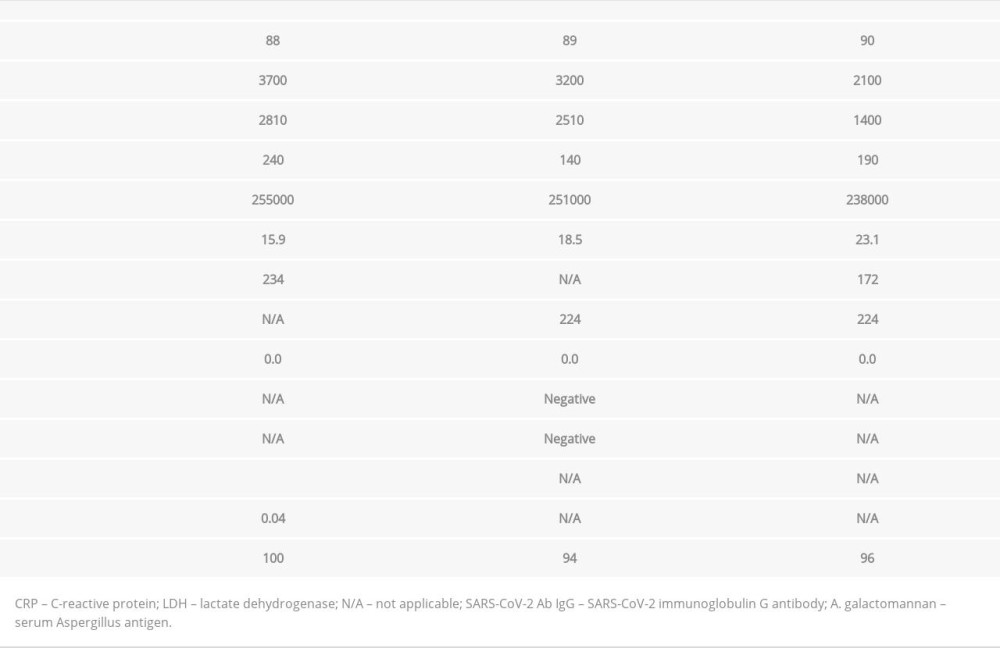 Table 1.. The patient’s laboratory diagnostics taken on 3 consecutive days: day 0 (admission day), day 1, and day 2. The patient received convalescent plasma on day 0.
Table 1.. The patient’s laboratory diagnostics taken on 3 consecutive days: day 0 (admission day), day 1, and day 2. The patient received convalescent plasma on day 0. Table 1.. The patient’s laboratory diagnostics taken on 3 consecutive days: day 0 (admission day), day 1, and day 2. The patient received convalescent plasma on day 0.
Table 1.. The patient’s laboratory diagnostics taken on 3 consecutive days: day 0 (admission day), day 1, and day 2. The patient received convalescent plasma on day 0. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








